Câu 1:
PT: \(Na_2CO_3+H_2SO_4\rightarrow Na_2SO_4+CO_2+H_2O\)
\(2NaHCO_3+H_2SO_4\rightarrow Na_2SO_4+2CO_2+2H_2O\)
Gọi: \(\left\{{}\begin{matrix}n_{Na_2CO_3}=x\left(mol\right)\\n_{NaHCO_3}=y\left(mol\right)\end{matrix}\right.\) ⇒ 106x + 84y = 9,1 (1)
Theo PT: \(n_{CO_2}=n_{Na_2CO_3}+n_{NaHCO_3}=x+y=\dfrac{2,016}{22,4}=0,09\left(mol\right)\left(2\right)\)
Từ (1) và (2) \(\Rightarrow\left\{{}\begin{matrix}x=0,07\left(mol\right)\\y=0,02\left(mol\right)\end{matrix}\right.\)
\(\Rightarrow\left\{{}\begin{matrix}m_{Na_2CO_3}=0,07.106=7,42\left(g\right)\\m_{NaHCO_3}=0,02.84=1,68\left(g\right)\end{matrix}\right.\)
Theo PT: \(n_{H_2SO_4}=n_{Na_2CO_3}+\dfrac{1}{2}n_{NaHCO_3}=0,08\left(mol\right)\)
\(\Rightarrow m_{H_2SO_4}=0,08.98=7,84\left(g\right)\Rightarrow m_{ddH_2SO_4}=\dfrac{7,84}{50\%}=15,68\left(g\right)\)
Câu 2:
Ta có: m bình 1 tăng = mH2O = 3,6 (g)
\(\Rightarrow n_{H_2O}=\dfrac{3,6}{18}=0,2\left(mol\right)\)
m bình 2 tăng = mCO2 = 13,2 (g)
\(\Rightarrow n_{CO_2}=\dfrac{13,2}{44}=0,3\left(mol\right)\)
PT: \(C_2H_4+3O_2\underrightarrow{t^o}2CO_2+2H_2O\)
\(2C_2H_2+5O_2\underrightarrow{t^o}4CO_2+2H_2O\)
Theo PT: \(\left\{{}\begin{matrix}n_{CO_2}=2n_{C_2H_4}+2n_{C_2H_2}=0,3\left(mol\right)\\n_{H_2O}=2n_{C_2H_4}+n_{C_2H_2}=0,2\left(mol\right)\end{matrix}\right.\)
\(\Leftrightarrow\left\{{}\begin{matrix}n_{C_2H_4}=0,05\left(mol\right)\\n_{C_2H_2}=0,1\left(mol\right)\end{matrix}\right.\)
\(\Rightarrow\left\{{}\begin{matrix}\%m_{C_2H_4}=\dfrac{0,05.28}{0,05.28+0,1.26}.100\%=35\%\\\%m_{C_2H_2}=65\%\end{matrix}\right.\)







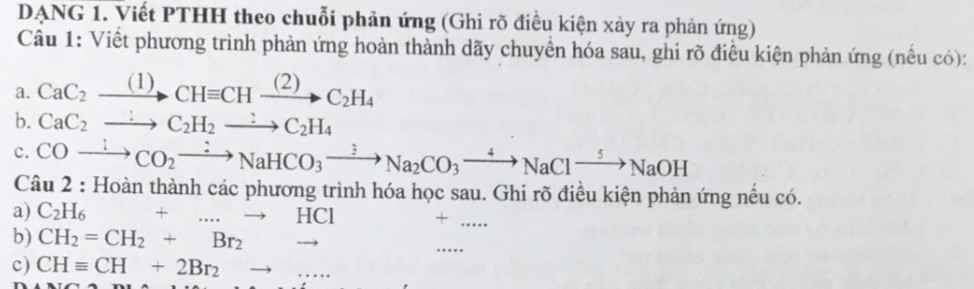 các bạn ko cần làm hết nha câu nào bt thôi thanks!
các bạn ko cần làm hết nha câu nào bt thôi thanks!







