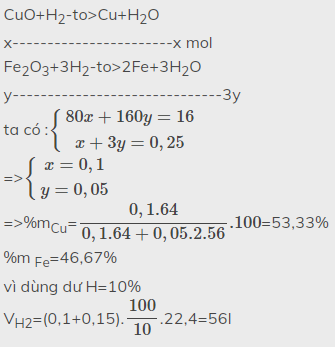CuO+H2-to>Cu+H2O
x-----------------x------x
Fe2O3+3H2->2Fe+3H2O
y-------------------2y--------3y
n H2O=\(\dfrac{4,5}{18}\)=0,25 mol
\(\left\{{}\begin{matrix}80x+160y=16\\x+3y=0,25\end{matrix}\right.\)
=>\(\left\{{}\begin{matrix}x=0,1\\y=0,05\end{matrix}\right.\)
=>m CuO=0,1.80=8g
=>m Fe2O3=0,05.160=8g
b)
%mCu=\(\dfrac{0,1.64}{0,1.64+0,05.2.56}\).100=53,3%
%m Fe=46,7%
c) lấy dư 10%
VH2=(0,1+3.0,05).22,4.\(\dfrac{110}{100}\)=6,16l