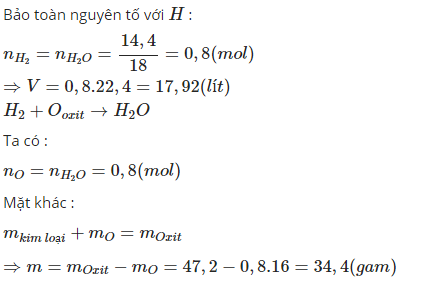n H2O=14,41814,418 =0,8 mol
⇒n H2=0,8 mol
n O=0,8 mol
⇒V H2(đktc)=0,8.22,4=17,92 l
theo đlbt khối lượng:
mkl+mO=m oxit
⇔mkl+0,8.16=47,2
⇔mkl=34,4 g
\(n_{H_2O}=n_{H_2}=n_{O\left(mất\right)}=\dfrac{14,4}{18}=0,8\left(mol\right)\\ \Rightarrow m=m_{kim.loại}=m_{hhoxit}-m_{O\left(mất\right)}=47,2-0,8.16=34,4\left(g\right)\\ V=V_{H_2\left(đktc\right)}=0,8.22,4=17,92\left(l\right)\)
\(n_{H_2O}=\dfrac{14,4}{18}=0,8\left(mol\right)\)
PTHH : CuO + H2 -> Cu + H2O
PTHH : FeO + H2 -> Fe + H2O
PTHH : Fe3O4 + 4H2 -> 3Fe + 4H2O
Ta thấy \(n_{H_2}=n_{H_2O}=0,8\left(mol\right)\)
\(V_{H_2}=0,8.22,4=17,92\left(l\right)\)
\(m_{H_2}=0,8.2=1,6\left(g\right)\)
Theo ĐLBTKL
\(m_{kimloại}=\left(m_{hỗnhợp}+m_{H_2}\right)-m_{H_2O}\\ \Rightarrow m_{kimloai}=\left(47,2+1,6\right)-14,4=34,4\left(g\right)\)