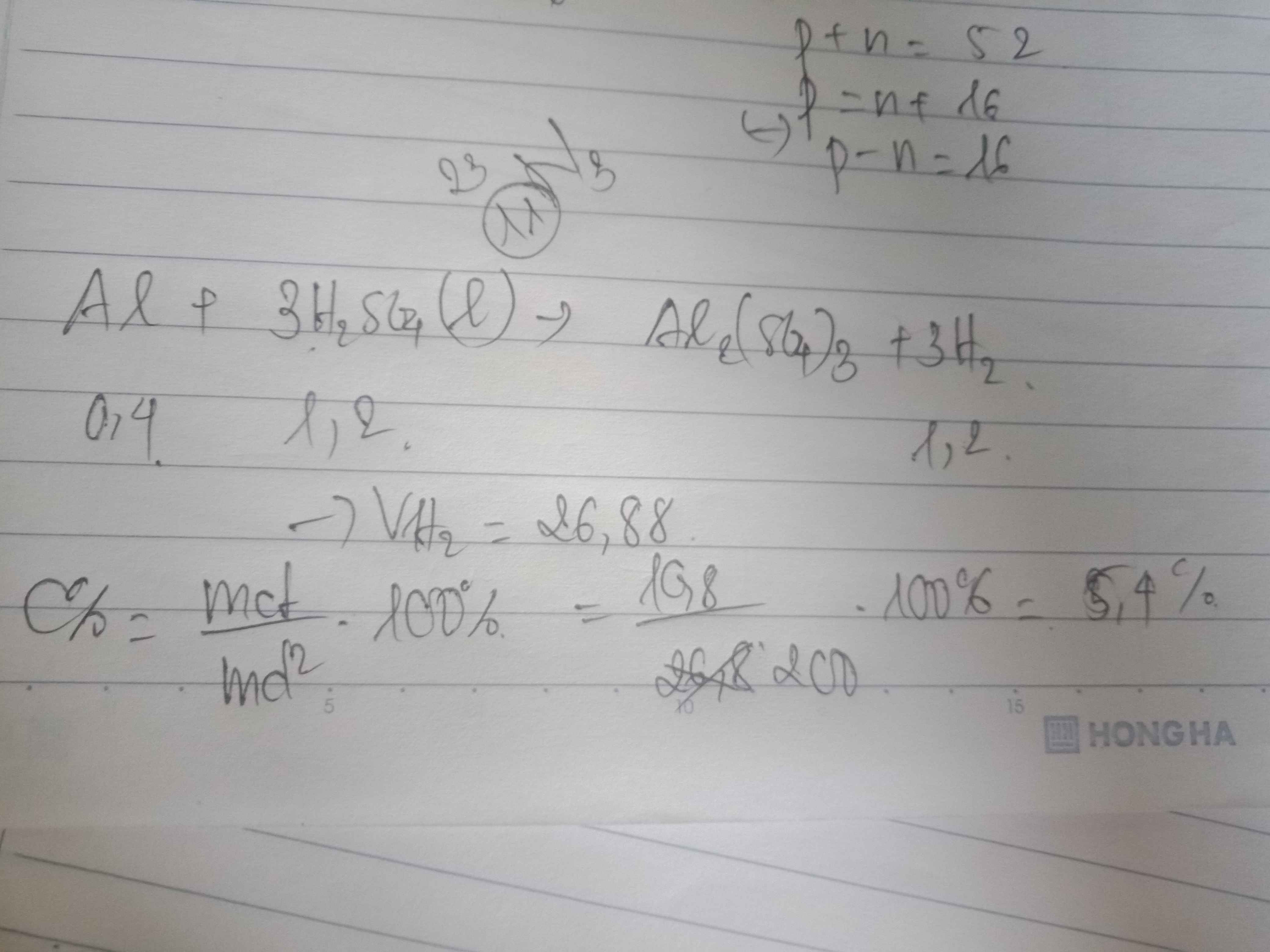a)b) \(n_{Al}=\dfrac{10,8}{27}=0,4\left(mol\right)\)
PTHH:
\(2Al+3H_2SO_4->Al_2\left(SO_4\right)_3+3H_2\)
0,4 --> 0,6 -->0,6 (mol)
--> \(n_{H_2}=0,6\left(mol\right)->V_{H_2}=n_{H_2}\cdot22,4=0,6\cdot22,4=13,44\left(lít\right)\)
--> \(n_{H_2SO_4}=0,6\left(mol\right)\) -> \(m_{H_2SO_4\left(ct\right)}=0,6\cdot98=58,8\left(g\right)\)
-> \(C\%_{H_2SO_4}=\dfrac{m\left(ct\right)}{m\left(dd\right)}\cdot100\%=\dfrac{58,8}{200}\cdot100\%=29,4\%\)