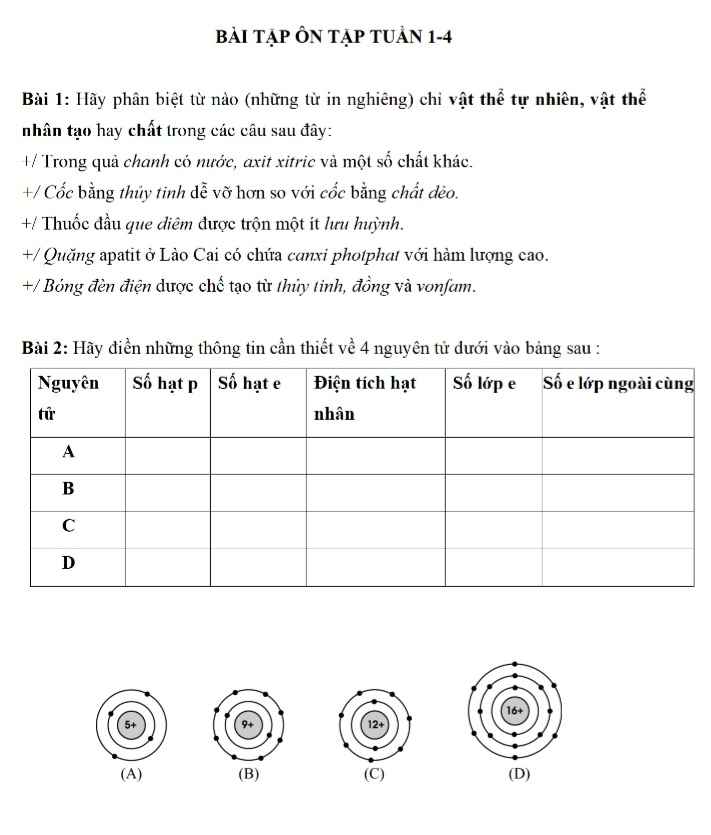
Bài 1:
PT: \(2H_2+O_2\underrightarrow{t^o}2H_2O\)
Xét tỉ lệ: \(\dfrac{2}{2}=\dfrac{1}{1}\), ta được pư vừa đủ.
Có: H% = 80% nên sau pư, hh Y gồm: \(\left\{{}\begin{matrix}V_{N_2}=3\left(l\right)\\V_{H_2}=2.20\%=0,4\left(l\right)\\V_{O_2}=1.20\%=0,2\left(l\right)\end{matrix}\right.\)
Ta có: \(M_X=\dfrac{\dfrac{3}{22,4}.28+\dfrac{2}{22,4}.2+\dfrac{1}{22,4}.32}{\dfrac{3}{22,4}+\dfrac{2}{22,4}+\dfrac{1}{22,4}}=20\left(g/mol\right)\)
\(M_Y=\dfrac{\dfrac{3}{22,4}.28+\dfrac{0,4}{22,4}.2+\dfrac{0,2}{22,4}.32}{\dfrac{3}{22,4}+\dfrac{0,4}{22,4}+\dfrac{0,2}{22,4}}=\dfrac{76}{3}\left(g/mol\right)\)
\(\Rightarrow d_{Y/X}=\dfrac{\dfrac{76}{3}}{20}\approx1,267\)

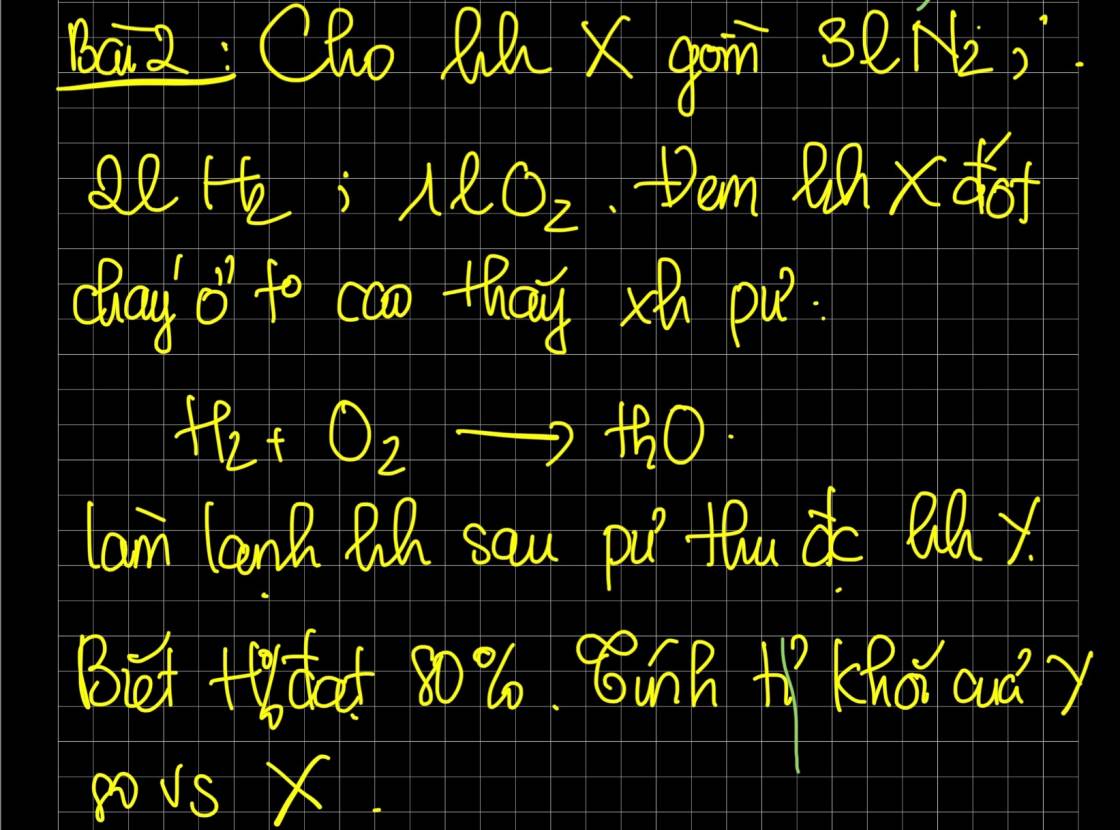







 help
help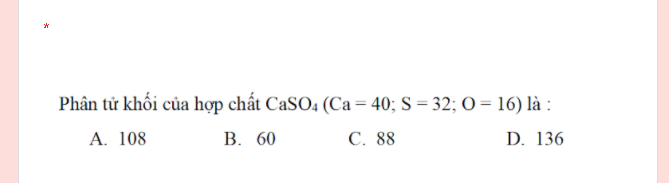 help
help  help
help