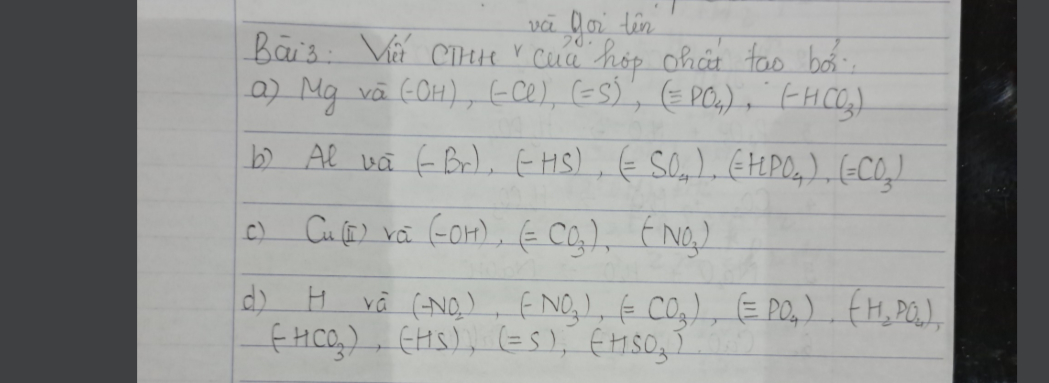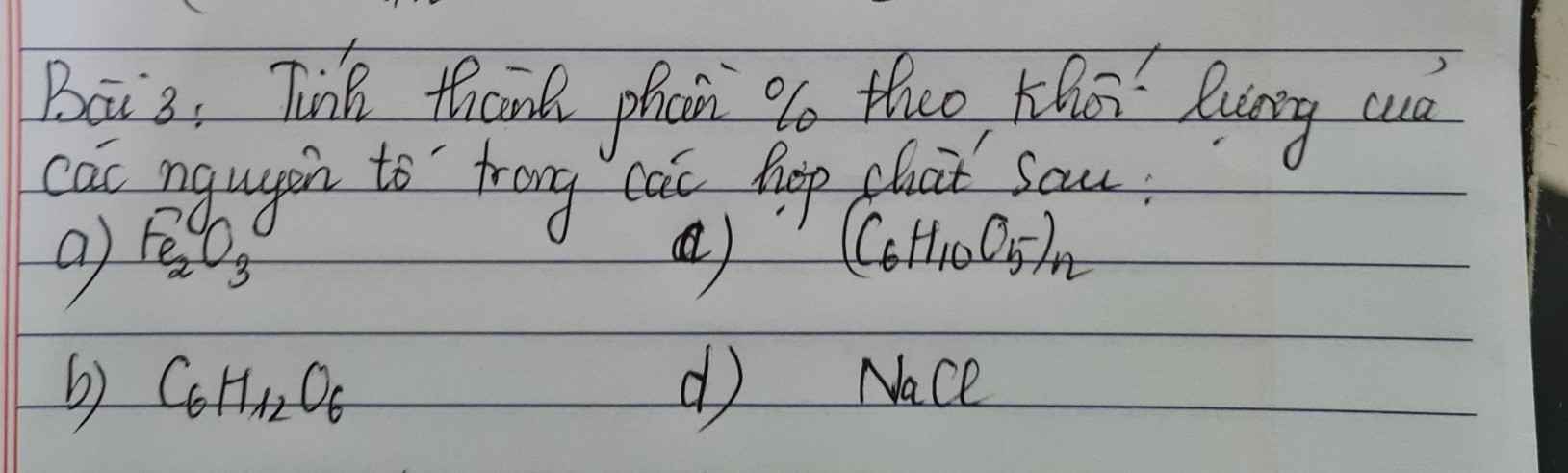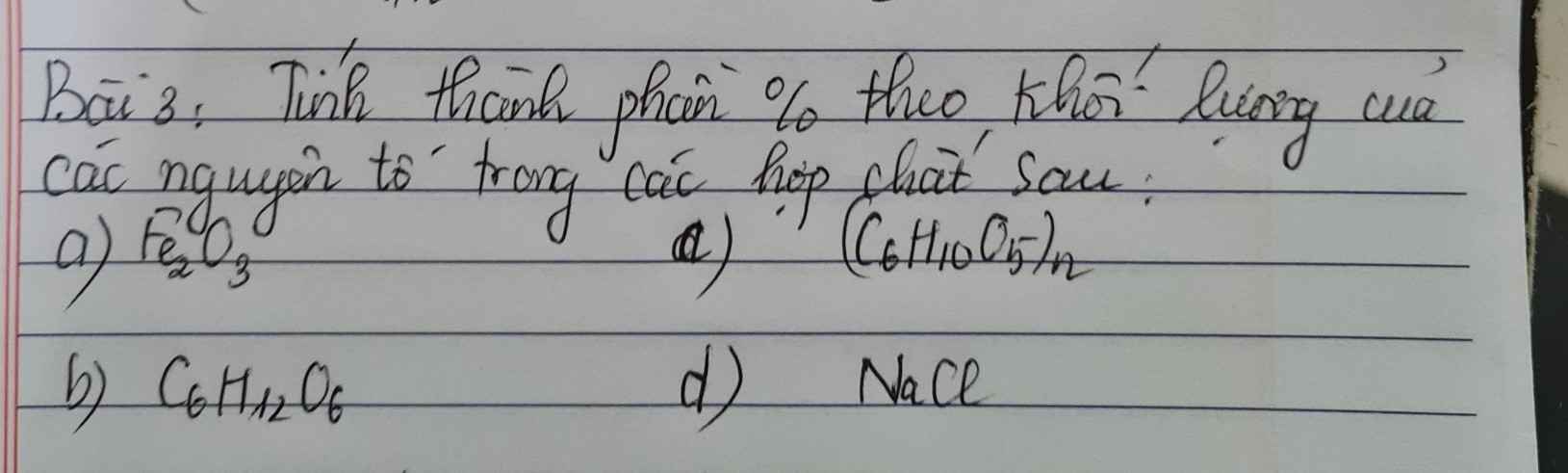Bài 3:
\(a,n_{Fe_3O_4}=\dfrac{2,32}{232}=0,01(mol)\\ PTHH:3Fe+2O_2\xrightarrow{t^o}Fe_3O_4\\ \Rightarrow n_{Fe}=0,03(mol);n_{O_2}=0,02(mol)\\ \Rightarrow m_{Fe}=0,03.56=1,68(g)\\ V_{O_2}=0,02.22,4=0,448(l)\\\)
\(b,PTHH:2KMnO_4\xrightarrow{t^o}K_2MnO_4+MnO_2+O_2\\ \Rightarrow n_{KMnO_4}=2n_{O_2}=0,04(mol)\\ \Rightarrow m_{KMnO_4}=0,04.158=6,32(g)\)