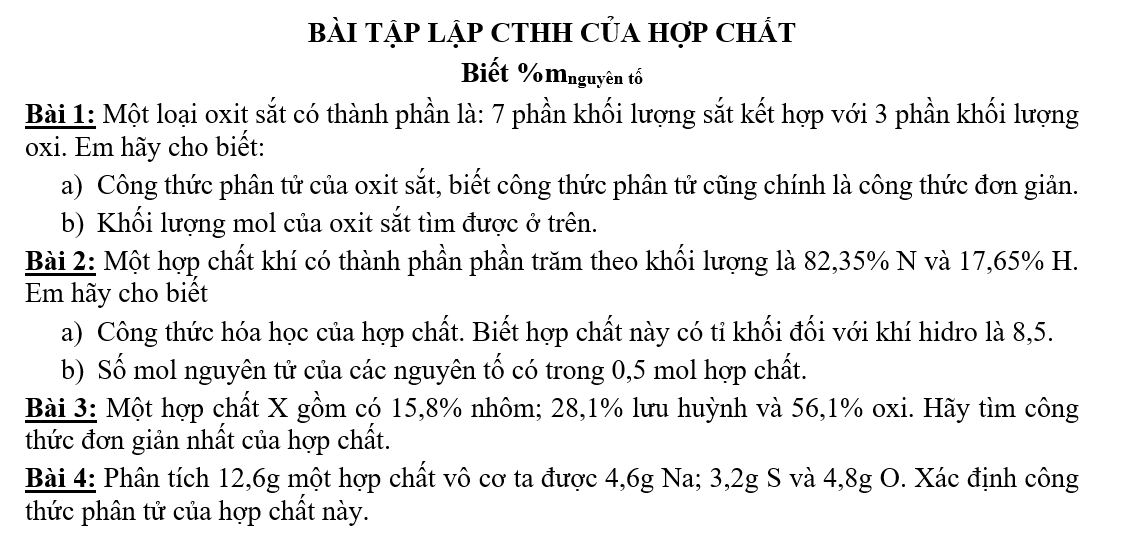
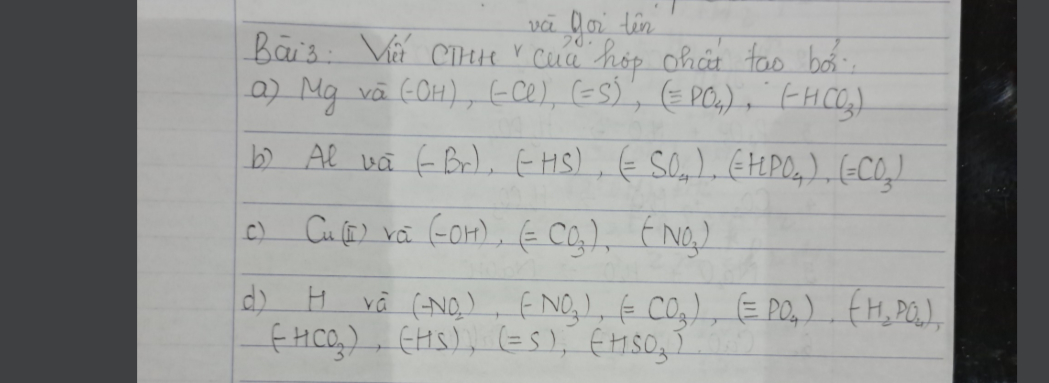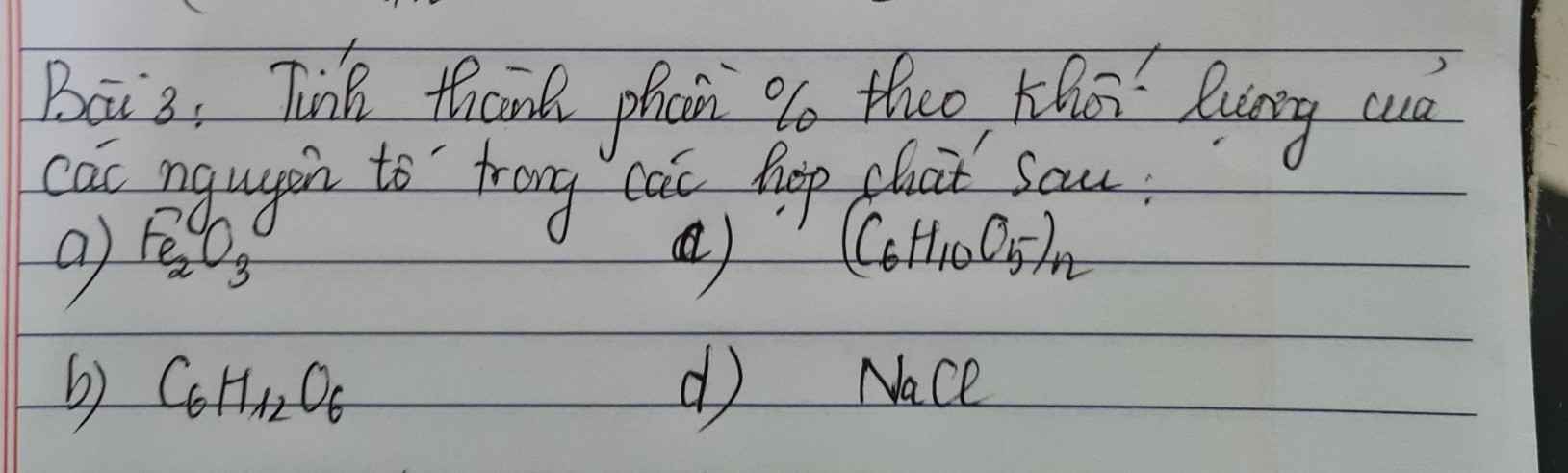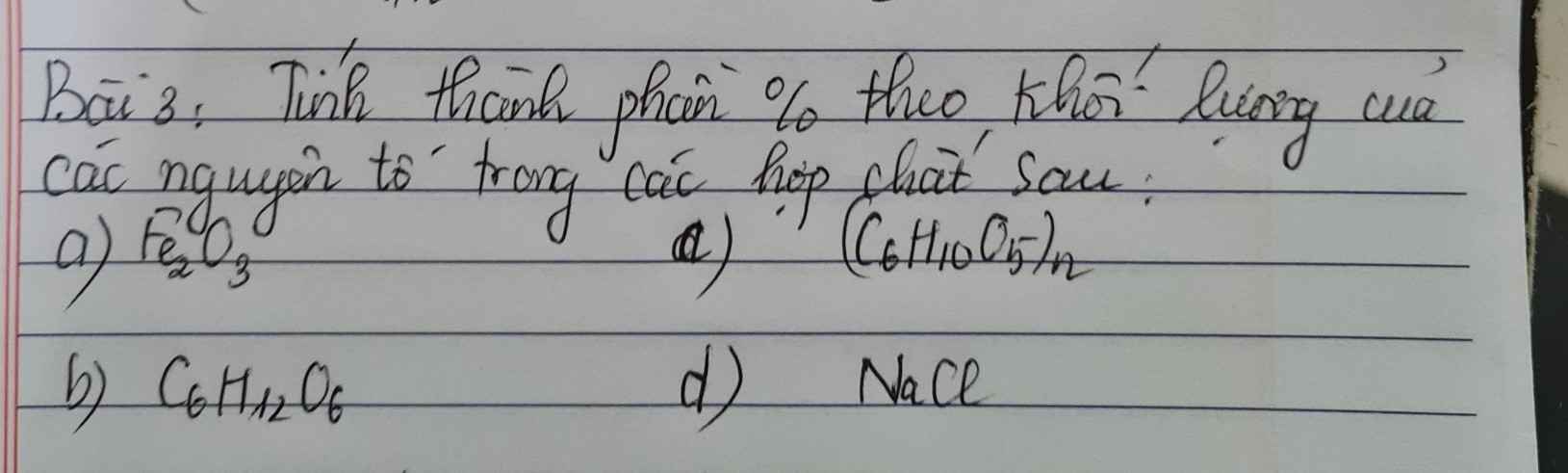Bài 5:
\(a,4P+5O_2\xrightarrow{t^o}2P_2O_5\\ b,n_{P}=\dfrac{3,1}{31}=0,1(mol)\\ \Rightarrow n_{O_2}=0,125(mol)\\ \Rightarrow V_{O_2}=0,125.22,4=2,8(l)\\ c,m_{O_2}=0,125.32=4(g)\\ \Rightarrow m_{P_2O_5}=m_{O_2}+m_{P}=4+3,1=7,1(g)\)
Bài 7:
\(a,n_{CO_2}=\dfrac{11}{44}=0,25(mol)\\ b,n_{H_2}=\dfrac{9.10^{23}}{6.10^{23}}=1,5(mol)\\ \Rightarrow V_{H_2}=1,5.22,4=33,6(l)\)
Bài 8:
\(a,n_{O_2}=\dfrac{67,2}{22,4}=3(mol)\\ b,Có 3.6.10^{23}=18.10^{23}\\ c,m_{O_2}=3.32=96(g)\\ d,n_{O_2}=\dfrac{3,2}{32}=0,1(mol)\\ \Rightarrow n_{N_2}=4n_{O_2}=0,4(mol)\\ \Rightarrow m_{N_2}=0,4.28=11,2(g)\)