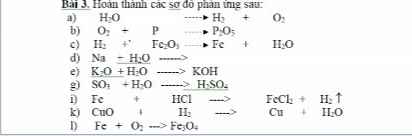
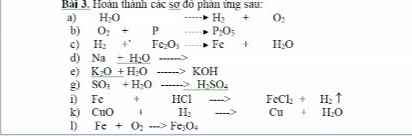
m NaCl = 200.11,6% = 23,2(gam)
$2NaCl + 2H_2O \xrightarrow{đpdd} 2NaOH + H_2 + Cl_2$
n H2 = 1,68/22,4 = 0,075(mol)
Theo PTHH :
n NaCl pư = n NaOH = 2n H2 = 0,15(mol)
n Cl2 = n H2 = 0,075(mol)
Sau phản ứng :
m NaCl = 23,2 - 0,15.58,5 = 14,425(gam)
m dd = 200 - 0,075.2 - 0,075.71 = 194,525(gam)
Ta có :
C% NaCl = 14,425/194,525 .100% = 7,42%
C% NaOH = 0,15.40/194,525 .100% = 3,08%