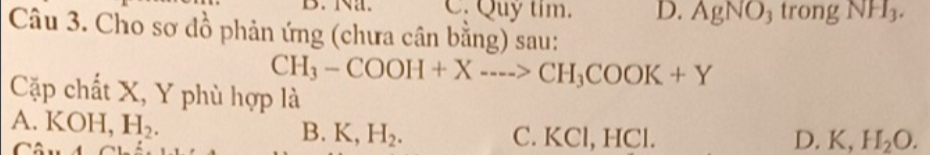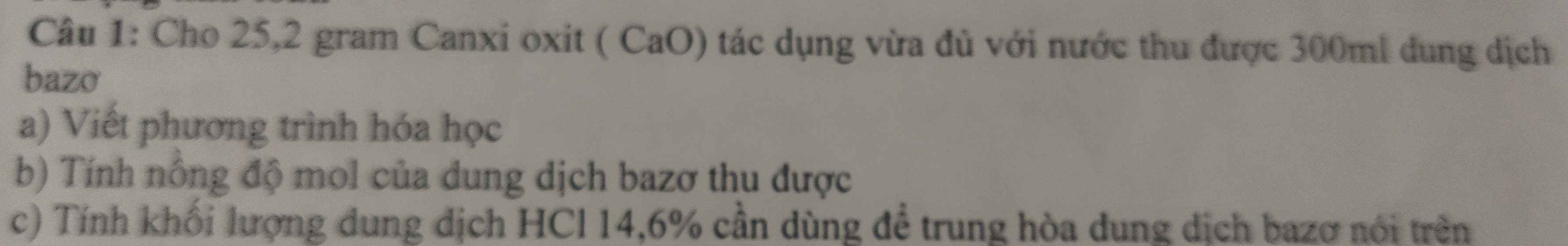Câu 3:
$a) \rm n_{Fe} = \dfrac{84}{56} = 1,5 (mol)$
PTHH:
$\rm Fe + 2HCl \rightarrow FeCl_2 + H_2$
Theo PT: $\rm n_{FeCl_2} = n_{Fe} = 1,5 (mol)$
$\rm m_{FeCl_2} = 1,5.127 = 190,5 (g)$
$\rm b)$ Theo PT: $\rm n_{KOH} = 2n_{FeCl_2} = 3 (mol)$
$\rm \Rightarrow C_{M(KOH)} = \dfrac{3}{\dfrac{200}{1000}} = 15M$
Đúng 2
Bình luận (1)