Bài 3.\(a.n_{CaCO_3}=\dfrac{7,5}{100}=0,075\left(mol\right)\\ CaCO_3+2HCl\rightarrow CaCl_2+CO_2+H_2O\\ b.n_{HCl}=2n_{CaCO_3}=0,15\left(mol\right)\\ \Rightarrow m_{ddHCl}=\dfrac{0,15.36,5}{7,3\%}=75\left(g\right)\\ c.n_{CO_2}=n_{CaCO_3}=0,075\left(mol\right)\\ m_{ddsaupu}=m_{CaCO_3}+m_{ddHCl}-m_{CO_2}=7,5+75-0,075.44=79,2\left(g\right)\\ n_{CaCl_2}=n_{CaCO_3}=0,075\left(mol\right)\\ \Rightarrow C\%_{CaCl_2}=\dfrac{0,075.111}{79,2}.100=10,51\%\)
Đúng 2
Bình luận (0)
Các câu hỏi tương tự
Mọi người giúp mình câu 1,câu 2, câu 3 với ạ
Giải giúp mình bài này với ạ giải câu c chi tiết xíu giúp mình với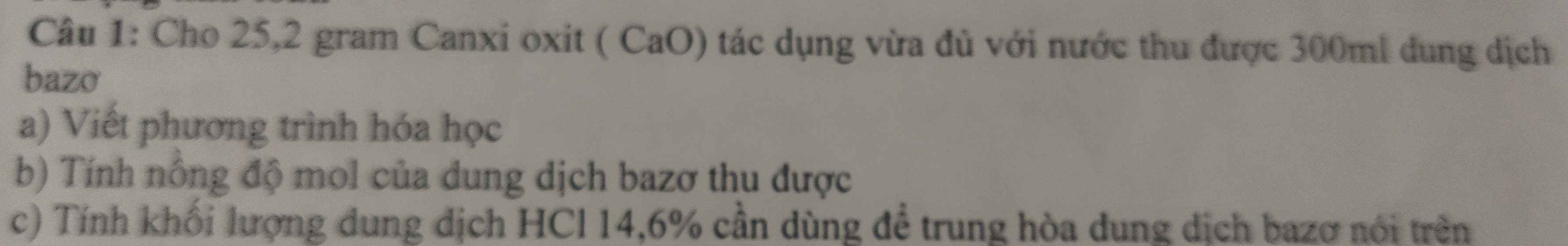
Mg giải giúp mình với ạ câu 5.
,6 ạ
Mọi người giúp mình câu d) với, mình đang cần gấp lắm ạ!!
Cảm ơn trước ạ
Giúp mình câu b với ạ, mình đang cần gấp!!!
Giúp mình câu 9 với ạ!
Giúp mình câu 5 với ạ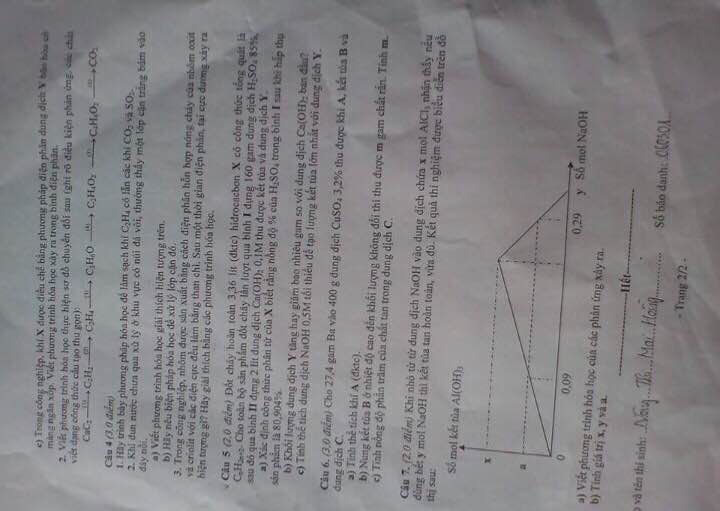
Giúp mình câu 4 với ạ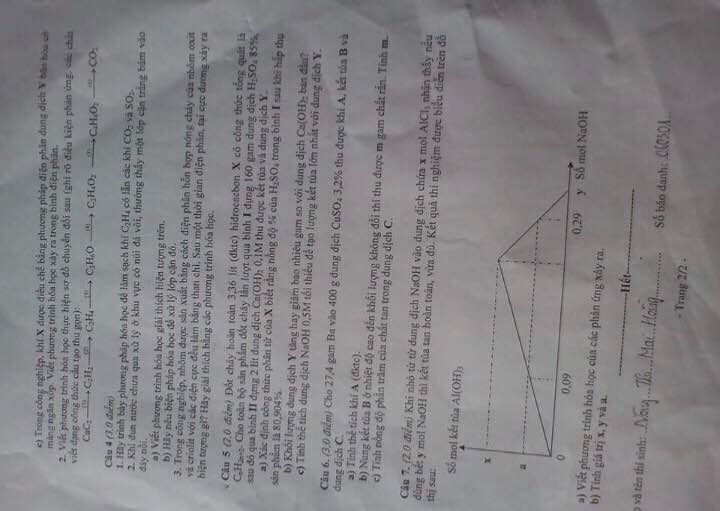
giải giúp mình câu d bài 15 với ạ:((












