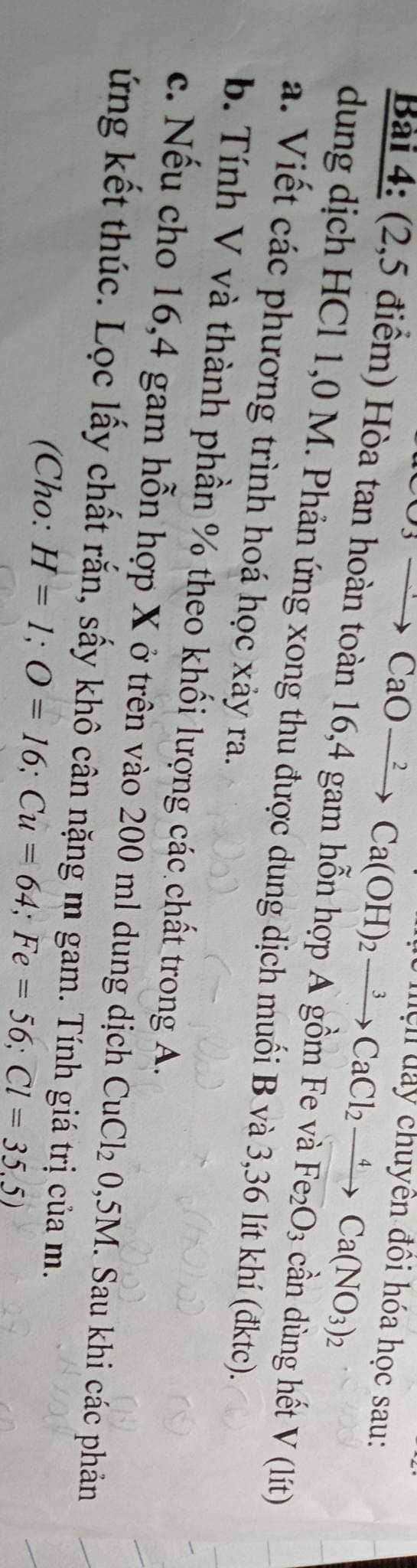a, PT: \(Fe+2HCl\rightarrow FeCl_2+H_2\)
\(Fe_2O_3+6HCl\rightarrow2FeCl_2+3H_2O\)
b, Ta có: \(n_{H_2}=0,15\left(mol\right)\)
Theo PT: \(n_{Fe}=n_{H_2}=0,15\left(mol\right)\)
\(\Rightarrow\left\{{}\begin{matrix}\%m_{Fe}=\dfrac{0,15.56}{16,4}.100\%\approx51,2\%\\\%m_{Fe_2O_3}\approx48,8\%\end{matrix}\right.\)
c, Ta có: mFe2O3 = 16,4 - 0,15.56 = 8 (g)
\(n_{CuCl_2}=0,2.0,5=0,1\left(mol\right)\)
PT: \(Fe+CuCl_2\rightarrow FeCl_2+Cu\)
Xét tỉ lệ: \(\dfrac{0,15}{1}>\dfrac{0,1}{1}\), ta được Fe dư.
Theo PT: \(n_{Fe\left(pư\right)}=n_{Cu}=n_{CuCl_2}=0,1\left(mol\right)\)
⇒ nFe (dư) = 0,05 (mol)
⇒ m = mFe2O3 + mFe dư + mCu = 8 + 0,05.56 + 0,1.64 = 17,2 (g)