\(n_{KMnO_4}=\dfrac{63,2}{158}=0,4\left(mol\right)\)
PTHH: 2KMnO4 --to--> K2MnO4 + MnO2 + O2
0,4 0,2
\(V_{O_2}=0,2.22,4=4,48\left(l\right)\)
\(n_{Zn}=\dfrac{39}{65}=0,6\left(mol\right)\)
PTHH: 2Zn + O2 --to--> 2ZnO
LTL: \(\dfrac{0,6}{2}>0,2\rightarrow Zn.dư\)
\(n_{KMnO_4}=\dfrac{63,2}{158}=0,4\left(mol\right)\)
\(pthh:2KMnO_4-t^o->K_2MnO_4+MnO_2+X\) =>X là O2
0,4 0,2
=> \(V_{O_2}=0,2.22,4=4,48\left(L\right)\)
\(n_{Zn}=\dfrac{39}{65}=0,6\left(mol\right)\)
pthh : \(2Zn+O_2-t^o->2ZnO \)
LTL : \(\dfrac{0,6}{2}< \dfrac{0,2}{1}\)
=> Zn dư => Không cháy hết

 giải giúp mình
giải giúp mình







 Giúp mình giải các câu hỏi trên trừ 2 câu khoanh tròn vì mình đã làm r giúp mik với :3 cảm ơn nhiều ( lưu ý : giải chi tiết vì mình đang bắt đầu học )
Giúp mình giải các câu hỏi trên trừ 2 câu khoanh tròn vì mình đã làm r giúp mik với :3 cảm ơn nhiều ( lưu ý : giải chi tiết vì mình đang bắt đầu học )



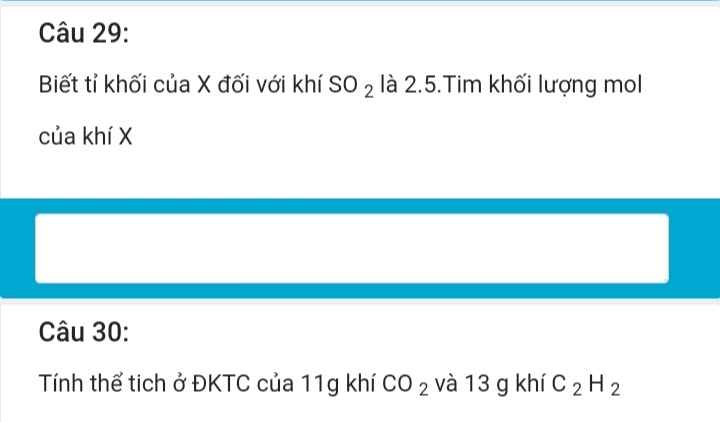

 Giải giúp mình câu 10 ik giải chi tiết nha
Giải giúp mình câu 10 ik giải chi tiết nha
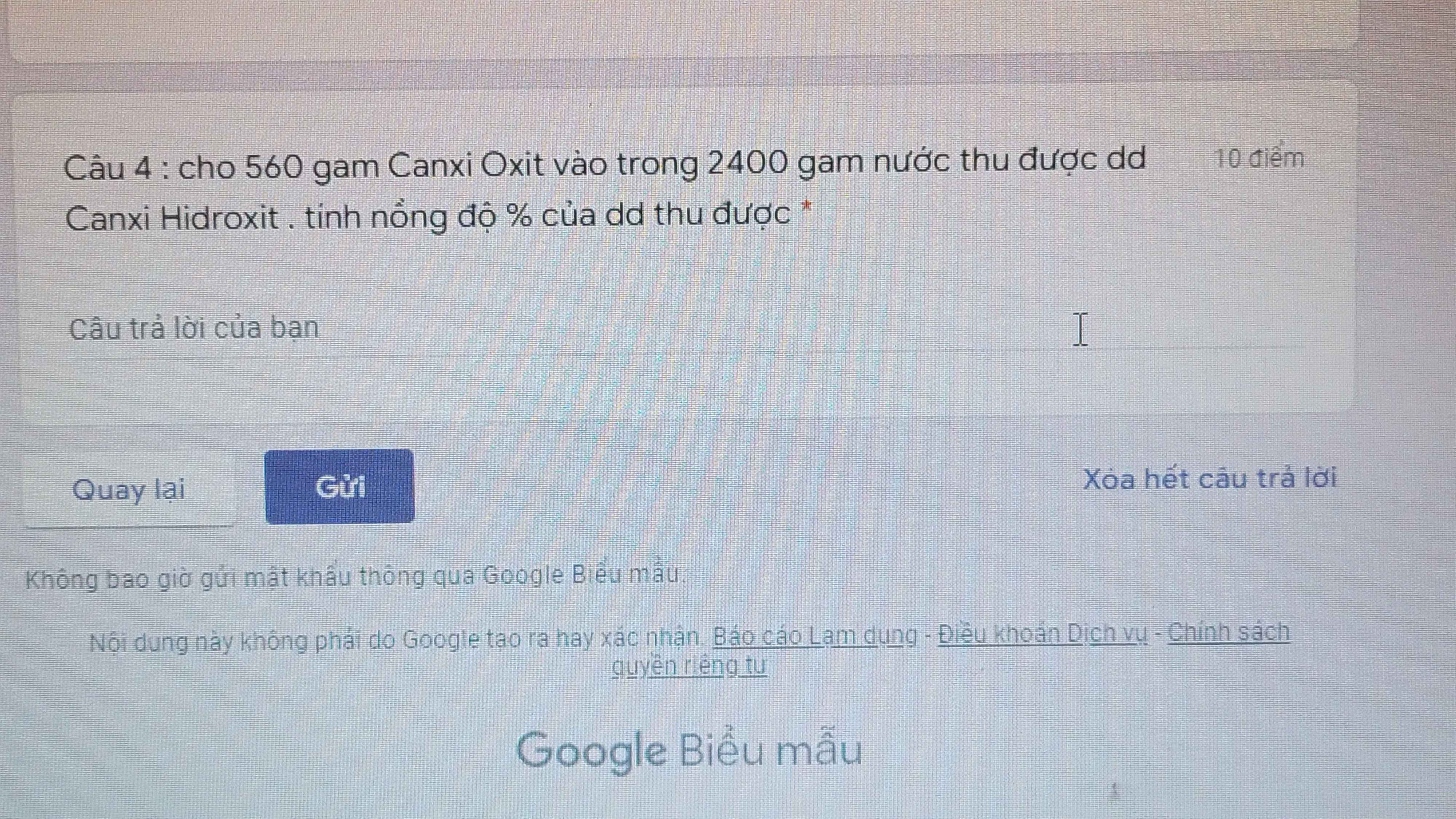
 giải chi tiết giúp mình c4 ạ. Mình cảm ơn!!!!!!!!
giải chi tiết giúp mình c4 ạ. Mình cảm ơn!!!!!!!!

