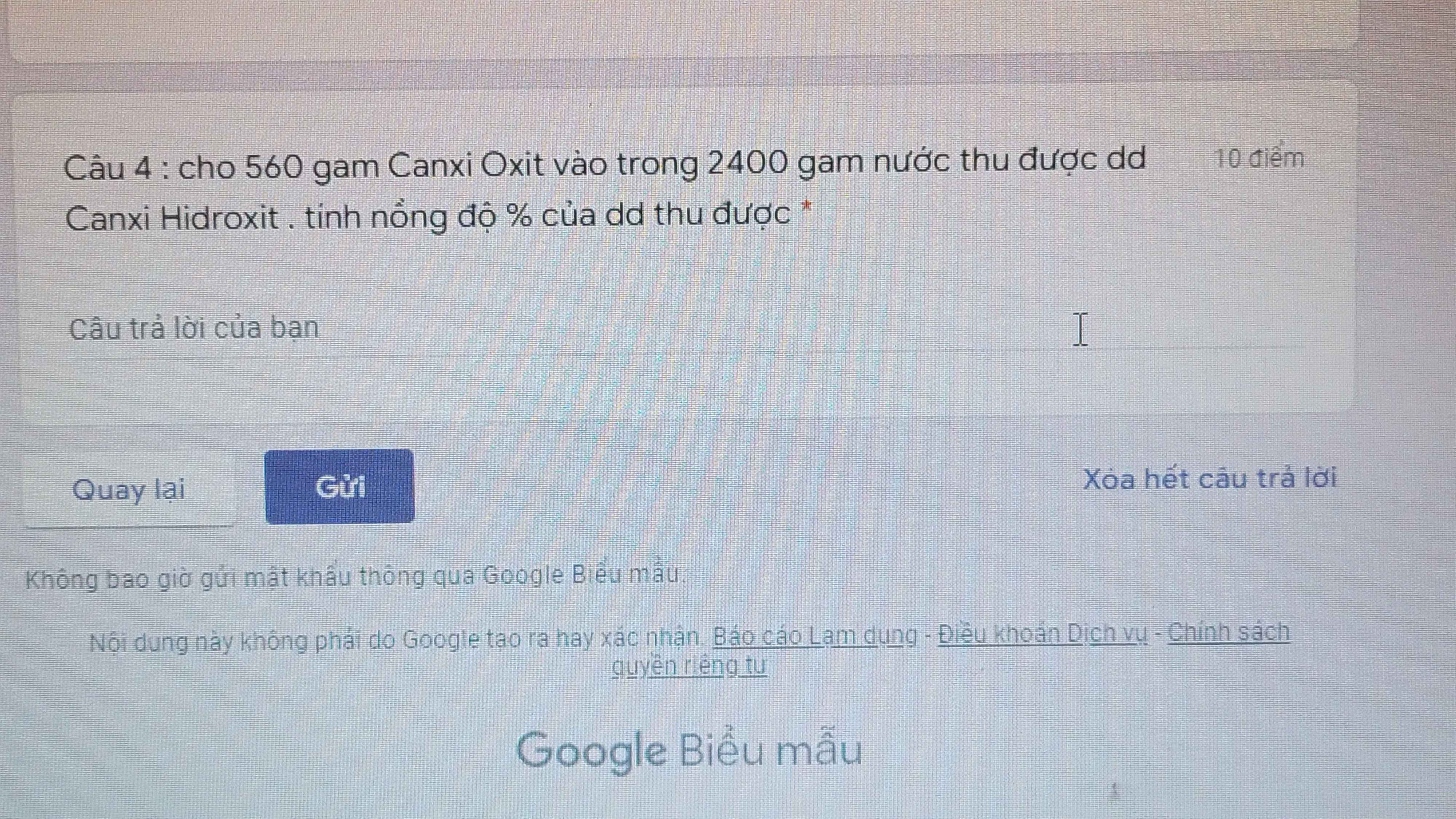
Câu 4.
\(n_{CaO}=\dfrac{560}{56}=10mol\)
\(n_{H_2O}=\dfrac{2400}{18}=\dfrac{400}{3}mol\)
\(CaO+H_2O\rightarrow Ca\left(OH\right)_2\)
10 \(\dfrac{400}{3}\) 10
\(m_{Ca\left(OH\right)_2}=10\cdot74=740g\)
\(m_{ddCa\left(OH\right)_2}=m_{CaO}+m_{H_2O}=560+2400=2960g\)
\(C\%=\dfrac{740}{2960}\cdot100\%=25\%\)








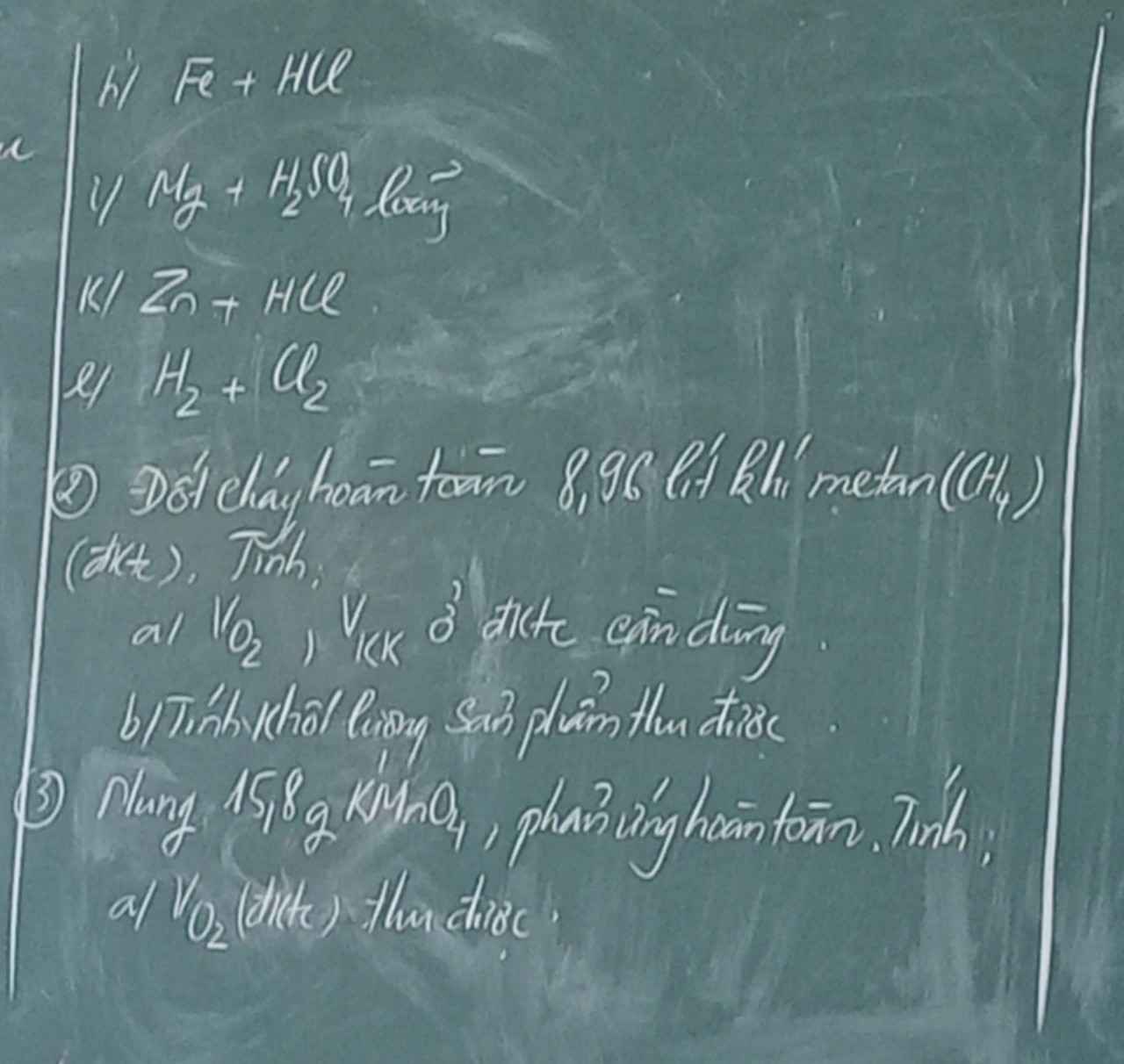

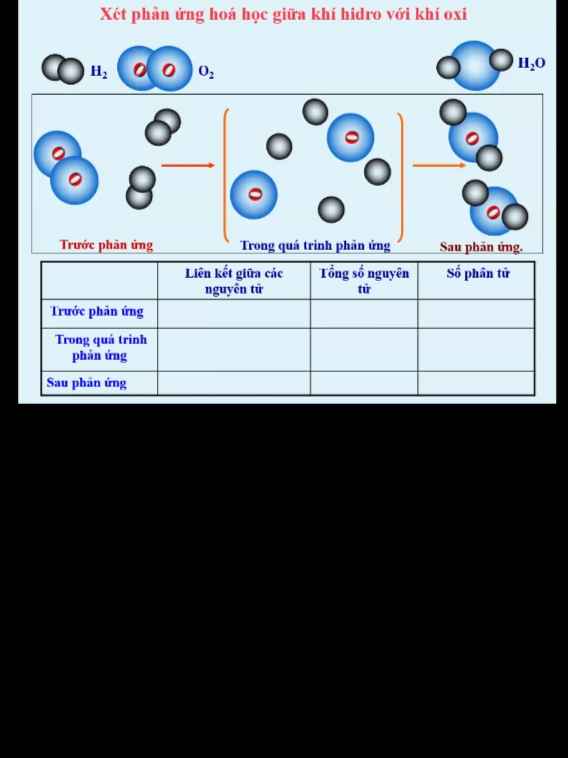




 Giúp mình giải các câu hỏi trên trừ 2 câu khoanh tròn vì mình đã làm r giúp mik với :3 cảm ơn nhiều ( lưu ý : giải chi tiết vì mình đang bắt đầu học )
Giúp mình giải các câu hỏi trên trừ 2 câu khoanh tròn vì mình đã làm r giúp mik với :3 cảm ơn nhiều ( lưu ý : giải chi tiết vì mình đang bắt đầu học )
 giải chi tiết giúp mình c4 ạ. Mình cảm ơn!!!!!!!!
giải chi tiết giúp mình c4 ạ. Mình cảm ơn!!!!!!!!





