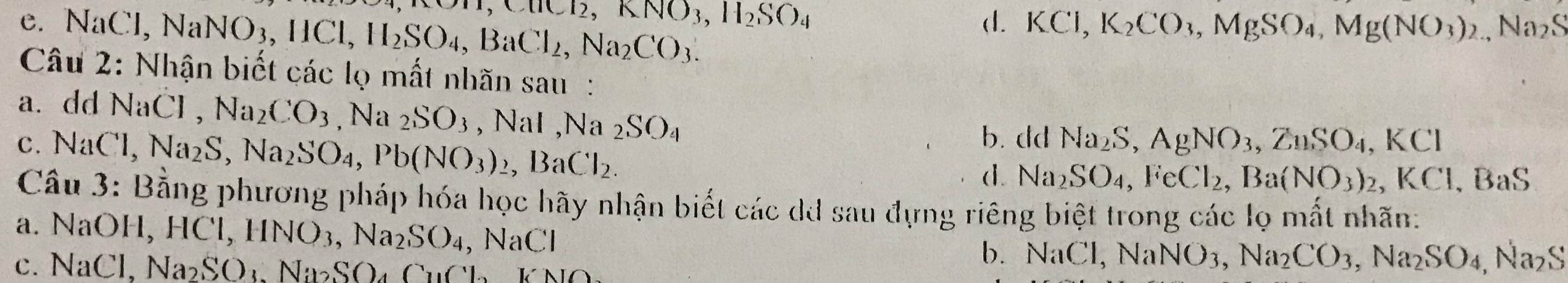\(n_{Fe}=\dfrac{5,6}{56}=0,1\left(mol\right)\\ n_S=\dfrac{0,8}{32}=0,025\left(mol\right)\)
PTHH: Fe + S --to--> FeS
LTL: \(0,1>0,025\rightarrow\) Fe dư
Theo pthh: \(n_{Fe\left(pu\right)}=n_{FeS}=n_S=0,025\left(mol\right)\)
\(\rightarrow\left\{{}\begin{matrix}m_{Fe\left(du\right)}=\left(0,1-0,025\right).56=4,2\left(g\right)\\m_{FeS}=0,025.88=2,2\left(g\right)\end{matrix}\right.\)
PTHH:
Fe + 2HCl ---> FeCl2 + H2
0,075 0,075
FeS + 2HCl ---> FeCl2 + H2S
0,025 0,025
\(\rightarrow\left\{{}\begin{matrix}\%V_{H_2}=\dfrac{0,075}{0,075+0,025}=75\%\\\%V_{H_2S}=100\%-75\%=25\%\end{matrix}\right.\)