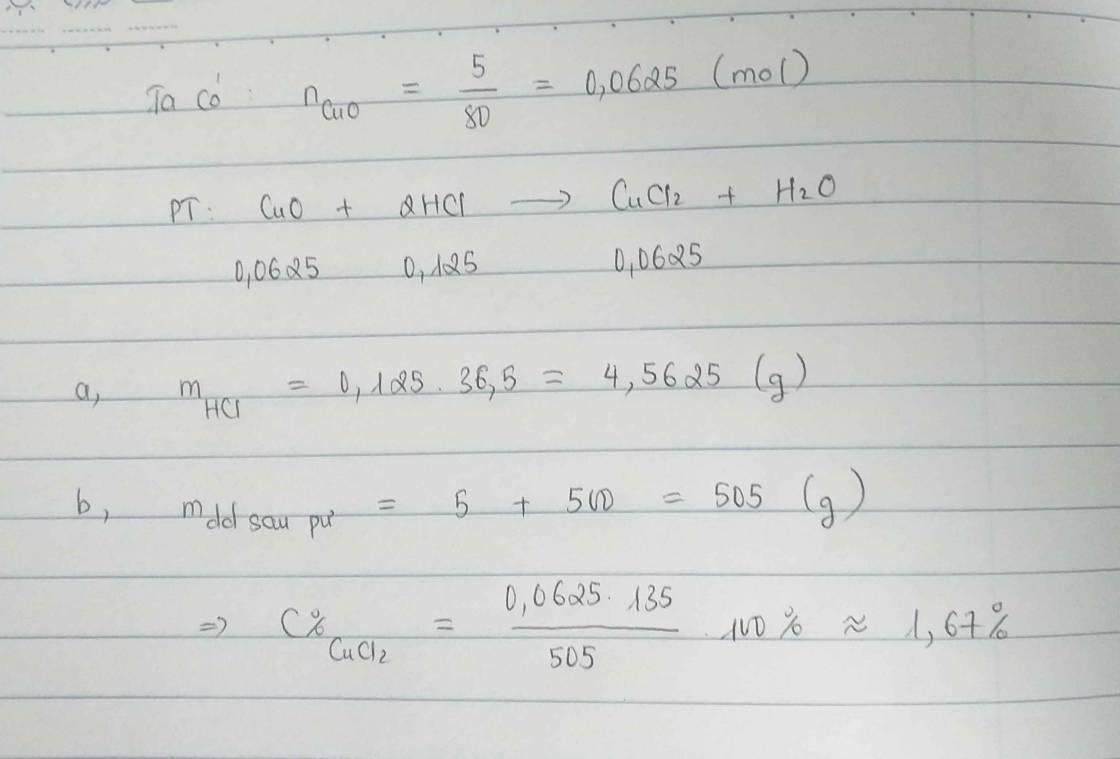a) $n_{CuO} = \dfrac{5}{80} = 0,0625(mol)$
$CuO + 2HCl \to CuCl_2 + H_2O$
$n_{HCl} = 2n_{CuO} = 0,125(mol)$
$m_{HCl} = 0,125.36,5 = 4,5625(gam)$
b) $m_{dd\ sau\ pư} = m_{CuO} + m_{dd\ HCl} = 5 + 500 = 505(gam)$
$C\%_{CuCl_2} = \dfrac{0,0625.135}{505}.100\% = 1,67\%$
\(CuO+2HCl\rightarrow CuCl_2+H_2O\)
\(n_{CuO}=\dfrac{5}{80}=\dfrac{1}{16}\left(mol\right)\)
Theo PTHH: \(n_{HCl}=2.n_{CuO}=\dfrac{1}{8}\left(mol\right)\)
\(m_{HCl}=\dfrac{1}{8}.36,5=\dfrac{73}{16}\left(g\right)\)
Theo PTHH: \(n_{CuCl_2}=n_{CuO}=\dfrac{1}{16}\left(mol\right)\)\(\Rightarrow m_{CuCl_2}=\dfrac{1}{16}.135=\dfrac{135}{16}\left(g\right)\)
\(C\%_{CuCl_2}=\dfrac{\dfrac{135}{16}}{500+5}.100\%\simeq1,67\%\)