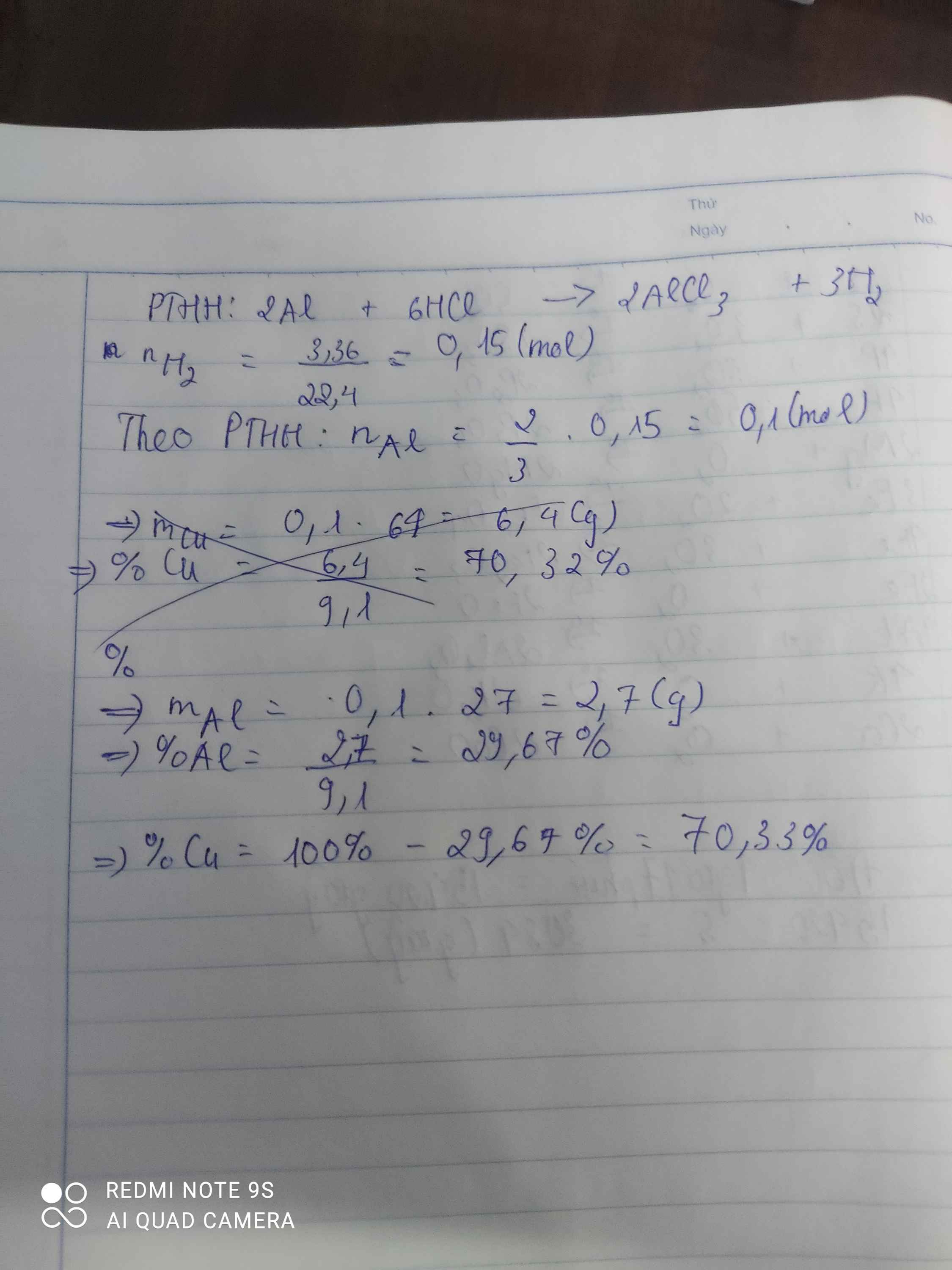\(n_{H_2}=\dfrac{3,36}{22,4}=0,15\left(mol\right)\)
PTHH: 2Al + 6HCl --> 2AlCl3 + 3H2
0,1<-------------------------0,15
=> \(\%m_{Al}=\dfrac{0,1.27}{9,1}.100\%=29,67\%=>\%m_{Cu}=100\%-29,67\%=70,33\%\)
\(n_{H_2}=\dfrac{3,36}{22,4}=0,15\left(mol\right)\)
Mà Cu k phản ứng hoàn toàn vs dd HCl
PTHH : 2Al + 6HCl -> 2AlCl3 + 3H2
0,1 mol ----------------------------- 0,15 mol
\(m_{Al}=0,1.27=2,7\left(g\right)\)
\(\%Al=\dfrac{2,7}{9,1}.100\%=29,6\%\)
\(\%Cu=100\%-29,6\%=70,4\%\)