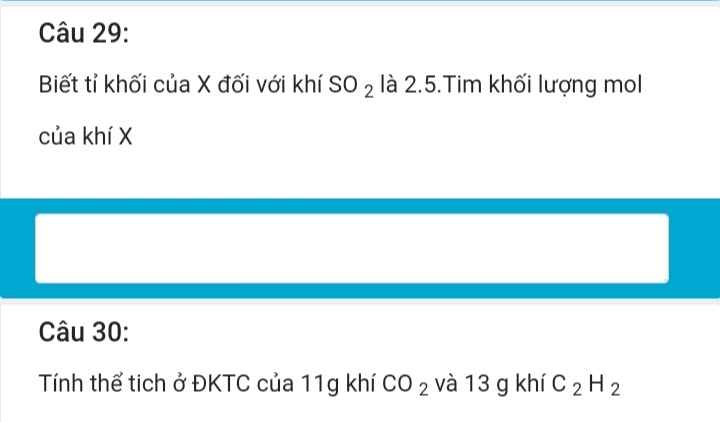
Bài 11:
a, Ta có: \(n_{H_2}=\dfrac{4,48}{22,4}=0,2\left(mol\right)\)
PT: \(A+H_2SO_4\rightarrow ASO_4+H_2\)
___0,2________________0,2 (mol)
\(\Rightarrow M_A=\dfrac{4,8}{0,2}=24\left(g/mol\right)\)
Vậy: A là Mg.
b, Theo PT: \(n_{H_2SO_4}=n_{H_2}=0,2\left(mol\right)\)
\(\Rightarrow V_{ddH_2SO_4}=\dfrac{0,2}{0,5}=0,4\left(l\right)\)
c, Theo PT: \(n_{MgSO_4}=n_{H_2}=0,2\left(mol\right)\)
\(\Rightarrow C_{M_{MgSO_4}}=\dfrac{0,2}{0,4}=0,5M\)
Bạn tham khảo nhé!
Bài 9:
Giả sử KL cần tìm là A.
PT: \(A+2HCl\rightarrow ACl_2+H_2\)
____0,3___0,6 (mol)
\(\Rightarrow M_A=\dfrac{7,2}{0,3}=24\left(g/mol\right)\)
Vậy: A là Magie (Mg)
Bạn tham khảo nhé!
Bài 10:
Ta có: \(n_{H_2}=\dfrac{8,96}{22,4}=0,4\left(mol\right)\)
PT: \(X+2HCl\rightarrow XCl_2+H_2\)
___0,4________________0,4 (mol)
\(\Rightarrow M_X=\dfrac{9,6}{0,4}=24\left(g/mol\right)\)
Vậy: X là Magie (Mg)
Bạn tham khảo nhé!
\(A+H_2SO_4\rightarrow ASO_4+H_2\)
Ta có: \(n_A=n_{H_2}=\dfrac{4,48}{22,4}=0,2\left(mol\right)\)
=> \(M_A=\dfrac{4,8}{0,2}=24\left(Mg\right)\)
\(n_A=n_{H_2}=\dfrac{4,48}{22,4}=0,2\left(mol\right)\)
\(n_{H_2SO_4}=n_{H_2}=0,2\left(mol\right)\)
=> \(V_{H_2SO_4}=\dfrac{0,2}{0,5}=0,4\left(l\right)\)
\(n_{MgSO_4}=n_{H_2}=0,2\left(mol\right)\)
\(CM_{MgSO_4}=\dfrac{0,2}{0,4}=0,5M\)







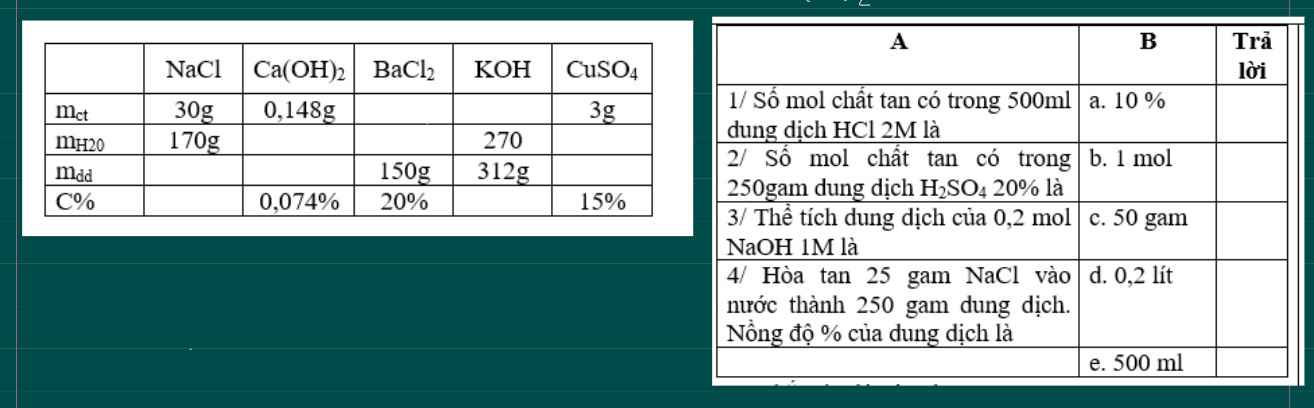 Các bạn giúp mình hoàn thiện 2 bảng naày với. Nhanh hộ minhf nha, mình đang cần gấp
Các bạn giúp mình hoàn thiện 2 bảng naày với. Nhanh hộ minhf nha, mình đang cần gấp




 mình đang cần gấp mng giúp mình nhé
mình đang cần gấp mng giúp mình nhé