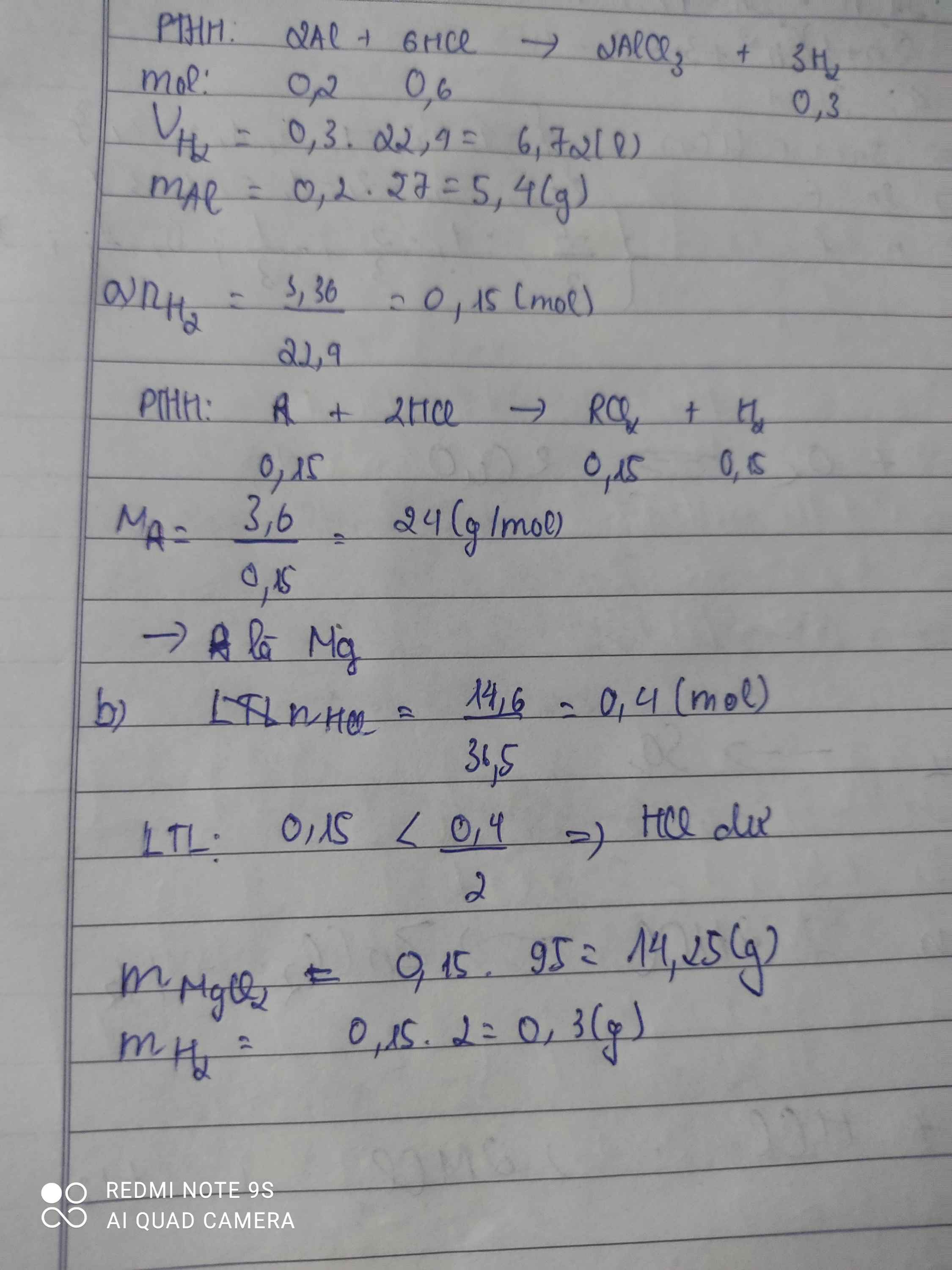\(a,A+2HCl\rightarrow ACl_2+H_2\\ n_{H_2}=\dfrac{3,36}{22,4}=0,15\left(mol\right)\Rightarrow n_A=n_{H_2}=0,15\left(mol\right)\\ \Rightarrow M_A=\dfrac{3,6}{0,15}=24\left(\dfrac{g}{mol}\right)\\ \Rightarrow A\left(II\right):Magie\left(Mg=24\right)\\ b,Mg+2HCl\rightarrow MgCl_2+H_2\\ n_{H_2}=\dfrac{14,6}{36,5}=0,4\left(mol\right)\\ Vì:\dfrac{0,15}{1}< \dfrac{0,4}{2}\Rightarrow HCldư\\ \Rightarrow Sau.p.ứ:MgCl_2,HCldư\\ n_{MgCl_2}=n_{Mg}=0,15\left(mol\right)\\ \Rightarrow m_{MgCl_2}=95.0,15=14,25\left(g\right)\\ n_{HCl\left(dư\right)}=0,4-0,15.2=0,1\left(mol\right)\\ \Rightarrow m_{HCl\left(dư\right)}=0,1.36,5=3,65\left(g\right)\\ m_{chất.sau}=3,65+14,25=17,9\left(g\right)\)
Đúng 4
Bình luận (2)
Các câu hỏi tương tự
Bài 24. Hòa tan 3,6g một kim loại A hóa trị II bằng một lượng dư axit HCl thu được 3,36 lít khí H2 (đktc). Xác định tên kim loại ABài 25. Hòa tan hoàn toàn 8,1g kim loại A hóa trị III trong dd HCl dư thu đucợ 10,08 lít khí H2 (đktc). Xác định tên A và m HCl đã dùng
Đọc tiếp
Bài 24. Hòa tan 3,6g một kim loại A hóa trị II bằng một lượng dư axit HCl thu được 3,36 lít khí H2 (đktc). Xác định tên kim loại A
Bài 25. Hòa tan hoàn toàn 8,1g kim loại A hóa trị III trong dd HCl dư thu đucợ 10,08 lít khí H2 (đktc). Xác định tên A và m HCl đã dùng
cho 8,4 gam một kim loại M hóa trị II vào dung dịch HCl 10% dư. Sau khi kim loại hòa tan hoàn toàn thu được 3,36 lít khí đo ở đktc và dung dịch A.
a. Xác định kim loại M
b.Để phản ứng hết dung dịch A cần 500ml dung dịch NaOH 1M. Tính nồng độ phần trăm các chất trong dung dịch A
Cho 5,4 gam kim loại nhôm tác dụng hoàn toàn với dung dịch axit clohiđric (HCl). Tính: a. Thể tích hiđro thu được ở đktc? b. Nếu dùng lượng khí Hiđro trên để khử vừa đủ một lượng oxit kim loại X hóa trị II thì thu được 19,5 gam kim loại. Tìm kim loại X.
Hòa tan hoàn toàn 6,5 gam kim loại kẽm bằng 1 lượng vừa đủ dung dịch axit clohidric (HCl), sau phản ứng thu được khí Hidro và muối kẽm clorua (ZnCl2).
a) Viết phương trình hóa học xảy ra?
b) Tính thể tích khí hidro thu được sau phản ứng ở đktc?
c) Tính khối lượng HCl đã phản ứng?
Cho 8,4 gam kim loại Magie (Mg) tác dụng hết với dung dịch axit clohidric HCl loãng, sau khi phản ứng kết thúc thu được V lít khí (đktc)
a) Viết phương trình phản ứng và gọi tên muối tạo thành
b) Tính giá trị V và khối lượng muối thu được
c) Nếu đốt chát lượng Magie trên trong bình chứa 2,24 lít khí O2(đktc) thì Magie có cháy hết không? Giải thích
Cho 4,8 gam kim loại magie tác dụng hoàn toàn với dung dịch axit HCl. Tính: a. Thể tích hiđro thu được ở đktc? c. Nếu dùng lượng khí Hiđro trên để khử vừa đủ một lượng oxit kim loại R hóa trị II thì thu được 12,8gam kim loại. Tìm kim loại R.
pls giải giúp vs ạ!!
B1: cho các chất sau: CuSO4, NaPO4, CaO. Gọi tên phân loại
B2: Điều chế khí hidro người ta tiến hành hòa tan hoàn toàn 9,75g kẽm bằng lượng vừa đủ 300ml dd oxit clohidric
1) Viết PTHH của pư
2) Tính V khí hidro thu được (đktc)
3) Tính Cm của dd axit clohidric đã dùng
B3: Cho sắt vào dd Axit Clohidric thu được muối sắt (II) clorua và giải phóng 0,448l hidro ở đktc
a) Nêu hiện tượng và viết PT
b) Tính khối lượng sắt pư
c) Nếu dùng lượng khí hidro thu được ở trên là 0,448 đem...
Đọc tiếp
pls giải giúp vs ạ!!
B1: cho các chất sau: CuSO4, NaPO4, CaO. Gọi tên phân loại
B2: Điều chế khí hidro người ta tiến hành hòa tan hoàn toàn 9,75g kẽm bằng lượng vừa đủ 300ml dd oxit clohidric
1) Viết PTHH của pư
2) Tính V khí hidro thu được (đktc)
3) Tính Cm của dd axit clohidric đã dùng
B3: Cho sắt vào dd Axit Clohidric thu được muối sắt (II) clorua và giải phóng 0,448l hidro ở đktc
a) Nêu hiện tượng và viết PT
b) Tính khối lượng sắt pư
c) Nếu dùng lượng khí hidro thu được ở trên là 0,448 đem khử đồng(II) oxit ở nhiệt độ cao thì thu được bao nhiêu gam đồng?
B4: Hòa tan 8,4g hỗn hợp hai kim loại Mg và Fe vào 400ml dd HCl nồng đồ 2M. CMR hỗn hợp kim loại tan hết
Hòa Tan hoàn toàn 7,2 g kim loại Mg vào 200 ml dd axit clohidric ( HCL ) sau phản ứng thu được magie clorua MgCl2 và khí hidro ( đktc )
a viết PTHH
b tính khối lượng magie clorua tạo thành
c tính nồng độ mol của dd axit đã dùng
Hoà tan hoàn toàn kim loại kẽm (Zn) vào dung dịch axit clohiđric (HCl) thu được kẽm clorua (ZnCl2) và 5,6 lít khí hiđro (đktc). a) Tính khối lượng kim loại và kẽm clorua trong phản ứng trên b) Cho khí hiđro vừa thu được qua sắt (II) oxit (FeO) đun nóng thu được kim loại sắt (Fe) và nước (H2O). Xác định khối lượng sắt thu được sau phản ứng.
Đọc tiếp
Hoà tan hoàn toàn kim loại kẽm (Zn) vào dung dịch axit clohiđric (HCl) thu được kẽm clorua (ZnCl2) và 5,6 lít khí hiđro (đktc). a) Tính khối lượng kim loại và kẽm clorua trong phản ứng trên b) Cho khí hiđro vừa thu được qua sắt (II) oxit (FeO) đun nóng thu được kim loại sắt (Fe) và nước (H2O). Xác định khối lượng sắt thu được sau phản ứng.