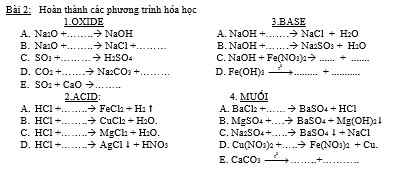
Câu 2:
\(n_{Fe}=\dfrac{1,12}{56}=0,02(mol)\\ n_{CuSO_4}=1,5.0,01=0,015(mol)\\ PTHH:Fe+CuSO_4\to FeSO_4+Cu\)
Vì \(\dfrac{n_{Fe}}{1}>\dfrac{n_{CuSO_4}}{1}\) nên \(Fe\) dư
Do đó X gồm \(Fe\) dư và \(Cu\)
\(\Rightarrow n_{Fe(dư)}=0,02-0,015=0,005(mol);n_{Cu}=0,015(mol)\\ \Rightarrow m_X=0,005.56+0,015.64=1,24(g)\\ \Rightarrow \%_{Fe(dư)}=\dfrac{0,005.56}{1,24}.100\%=22,58\%\\ \Rightarrow \%_{Cu}=100\%-22,58\%=77,42\%\\ b,PTHH:FeSO_4+2KOH\to Fe(OH)_2\downarrow+K_2SO_4\\ \Rightarrow n_{KOH}=2n_{FeSO_4}=2n_{Cu}=0,03(mol)\\ \Rightarrow V_{dd_{KOH}}=\dfrac{0,03}{1}=0,03(l)\)