\(n_{KClO_3}=\dfrac{5,5125}{122,5}=0,045\left(mol\right)\\ 2KClO_3\rightarrow\left(t^o\right)2KCl+3O_2\uparrow\\ n_{O_2}=\dfrac{3}{2}.0,045=0,0675\left(mol\right)\\ 2Cu+O_2\rightarrow\left(t^o\right)2CuO\\ n_{CuO}=2.0,0675=0,135\left(mol\right)\\ m_{r\text{ắn}}=m_{CuO}=0,135.80=10,8\left(g\right)\)
Đúng 1
Bình luận (0)
Các câu hỏi tương tự
Giúp mình với ạ! Khi giải ghi lời giải chi tiết giúp mình nka 💜💜
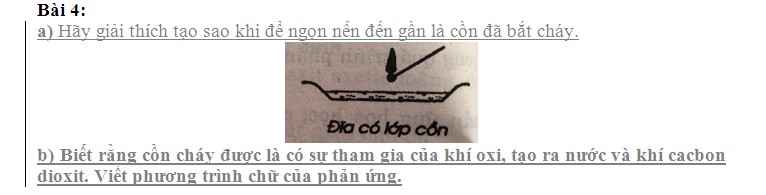 mấy anh chị giải chi tiết giùm em,em cần lời giải chi tiết ạ em xin cảm ơn
mấy anh chị giải chi tiết giùm em,em cần lời giải chi tiết ạ em xin cảm ơn
cho mình xin cách giải chi tiết ạ

cho mình xin cách giải chi tiết ạ

Một oxit của Crom là Cr2O3. Muối trong đó Crom có hóa trị tương ứng là .
Mình cần lời giải chi tiết nha. giúp mình với mai thi rầu :(((
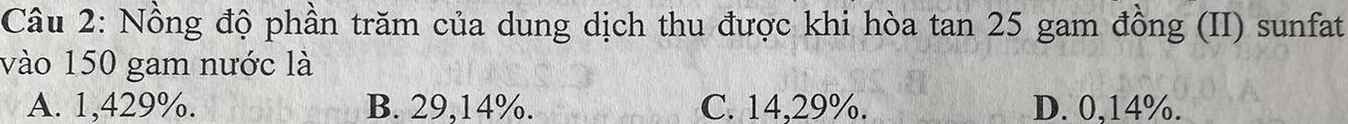 giải chi tiết giúp mình với ạ!!!!!!!!!
giải chi tiết giúp mình với ạ!!!!!!!!!
 giải chi tiết giúp mình c4 ạ. Mình cảm ơn!!!!!!!!
giải chi tiết giúp mình c4 ạ. Mình cảm ơn!!!!!!!!
 Giải giúp mình câu 10 ik giải chi tiết nha
Giải giúp mình câu 10 ik giải chi tiết nha
Hợp chất của nguyên tố X với S là X2S3 và hợp chất của nguyên tố Y với H là YH3. Công thức hóa học hợp chất của X với Y là.
Mình cần lời giải chi tiết ạ. Giúp mình với :(((









