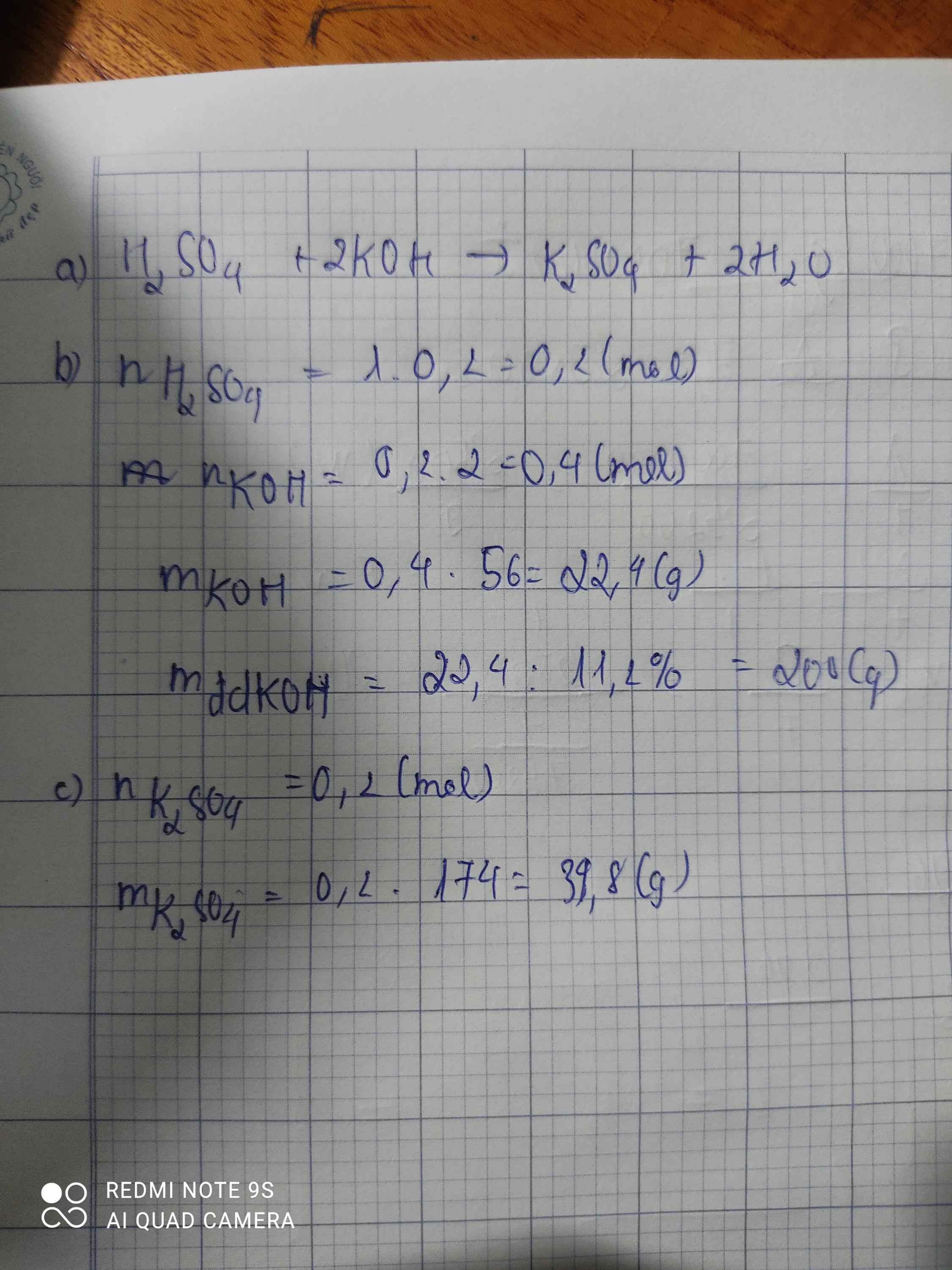\(n_{H_2SO_4}=0,2.1=0,2\left(mol\right)\\ a,PTHH:H_2SO_4+2KOH\rightarrow K_2SO_4+2H_2O\\ b,n_{KOH}=2.n_{H_2SO_4}=2.0,2=0,4\left(mol\right)\\ m_{ddKOH}=\dfrac{0,4.56.100}{11,2}=200\left(g\right)\\ c,n_{K_2SO_4}=n_{H_2SO_4}=0,2\left(mol\right)\\ \Rightarrow m_{K_2SO_4}=174.0,2=34,8\left(g\right)\)
H2SO4+2KOH->K2SO4+2H2O
0,2---------0,4---------0,2 mol
n H2SO4=0,2 mol
=>m KOH =0,4.56=22,4g
=>mdd=200g
c.mK2SO4=0,2.174=34,8g