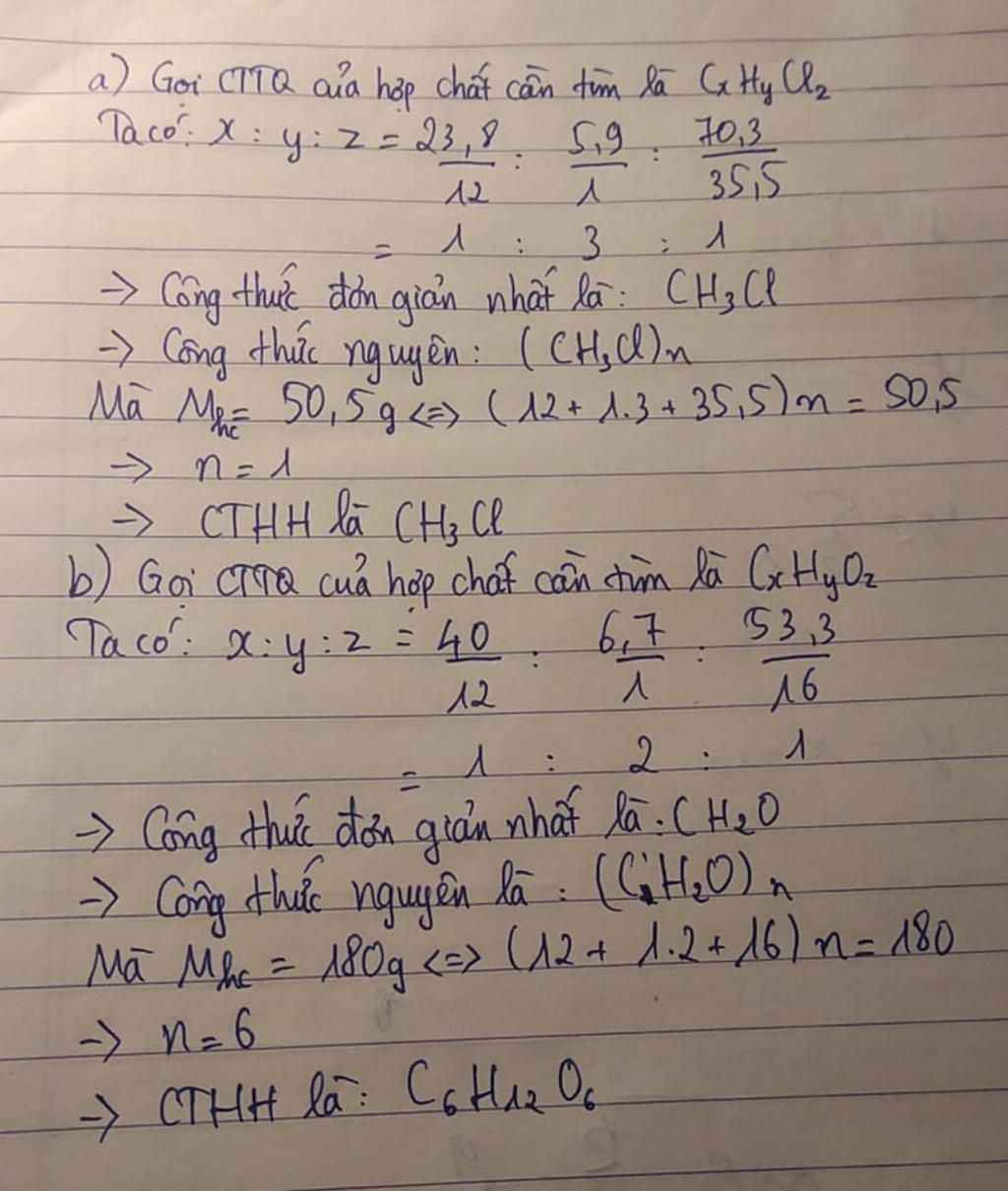\(m_C=\dfrac{50,5.23,8\%}{100\%}=12\left(g\right)\\ n_C=\dfrac{12}{12}=1\left(mol\right)\\ m_H=\dfrac{50,5.5,9\%}{100\%}=3\left(g\right)\\ n_C=\dfrac{3}{1}=3\left(mol\right)\\ m_{Cl}=50,5-12-3=35,5\left(g\right)\\ n_{Cl}=\dfrac{35,5}{35,5}=1\left(mol\right)\\ =>CTHH:CH_3Cl\)
a,
\(nC=\dfrac{\dfrac{23,8.50,5}{100}}{12}=1mol\)
\(nH=\dfrac{\dfrac{5,9.50,5}{100}}{1}=3mol\)
\(nCl=\dfrac{\dfrac{70,3.50,5}{100}}{35,5}=1mol\)
cthh :\(CH_3Cl\)
b,
\(nC=\dfrac{\dfrac{40.180}{100}}{12}=6mol\)
\(nH=\dfrac{\dfrac{6,7.180}{100}}{1}=12mol\)
\(nO=\dfrac{\dfrac{53,3.180}{100}}{16}=6mol\)
cthh:\(C_6H_{12}O_6\)