\(n_{NaOH}=\dfrac{100.14,4\%}{100\%.40}=0,36mol\\
T=\dfrac{0,36}{0,28}\\
\Rightarrow1< T< 2\)
Phản ứng tạo 2 muối
\(n_{Na_2CO_3}=a;n_{NaHCO_3}=b\\
2NaOH+CO_2\rightarrow Na_2CO_3+H_2O\\
NaOH+CO_2\rightarrow NaHCO_3\\
\Rightarrow\left\{{}\begin{matrix}2a+b=0,36\\a+b=0,28\end{matrix}\right.\\
\Rightarrow a=0,08;b=0,2\\
C_{\%Na_2CO_3}=\dfrac{0,08.106}{0,28.44+100}\cdot100\%=7,55\%\\
C_{\%NaHCO_3}=\dfrac{0,2.84}{0,28.44+100}\cdot100\%=14,96\%\%\)
Đúng 2
Bình luận (0)
Các câu hỏi tương tự
Sục 0,25 mol khí CO2 vào 200 ml dung dịch chứa Ba(OH)2 0,2M và NaOH 1M, sau khi phản ứng kết thúc, thu được dung dịch X. Dung dịch X chứa các chất tan là? A. Na2CO3 ,NaHCO3 B. Na2CO3 C. Na2CO3 , NaOH D. NaHCO3 , Ba(HCO3)2
Đọc tiếp
Sục 0,25 mol khí CO2 vào 200 ml dung dịch chứa Ba(OH)2 0,2M và NaOH 1M, sau khi phản ứng kết thúc, thu được dung dịch X. Dung dịch X chứa các chất tan là?
A. Na2CO3 ,NaHCO3
B. Na2CO3
C. Na2CO3 , NaOH
D. NaHCO3 , Ba(HCO3)2
Đốt cháy hoàn toàn m gam hợp chất X của photpho cần
m
17
mol oxi, sau phản ứng chỉ thu được P2O5 và
13
,
5
m
17
gam H2O. Cho toàn bộ sản phẩm cháy vào 125 gam dung dịch NaOH 16% thu được dung dịch B chỉ chứa hai muối NaH2PO4 và Na2HPO4 có nồng độ phần trăm bằng nhau. Giá trị của m là A. 24,35 B. 11...
Đọc tiếp
Đốt cháy hoàn toàn m gam hợp chất X của photpho cần m 17 mol oxi, sau phản ứng chỉ thu được P2O5 và 13 , 5 m 17 gam H2O. Cho toàn bộ sản phẩm cháy vào 125 gam dung dịch NaOH 16% thu được dung dịch B chỉ chứa hai muối NaH2PO4 và Na2HPO4 có nồng độ phần trăm bằng nhau. Giá trị của m là
A. 24,35
B. 11,66
C. 13,6
D. 11,9
Tiến hành các thí nghiệm sau :(a) Cho dung dịch chưa 4a mol HCl vào dung dịch chứa a mol NaAlO2(b) Cho Al2O3 dư vào lượng dư dung dịch NaOH(c) Sục khí CO2 đến dư vào dung dịch Ba(OH)2(d) Cho Fe vào dung dịch Fe2(SO4)3 dư(e) Cho dung dịch chứa a mol KHSO4 vào dung dịch chứa a mol NaHCO3(g) Cho Mg dư vào dung dịch HNO3 (phản ứng không thu được chất khí )Sau khi các phản ứng xảy ra hoàn toàn thì số thí nghiệm thu được dung dịch chứa hai muối là A. 3 B. 2 ...
Đọc tiếp
Tiến hành các thí nghiệm sau :
(a) Cho dung dịch chưa 4a mol HCl vào dung dịch chứa a mol NaAlO2
(b) Cho Al2O3 dư vào lượng dư dung dịch NaOH
(c) Sục khí CO2 đến dư vào dung dịch Ba(OH)2
(d) Cho Fe vào dung dịch Fe2(SO4)3 dư
(e) Cho dung dịch chứa a mol KHSO4 vào dung dịch chứa a mol NaHCO3
(g) Cho Mg dư vào dung dịch HNO3 (phản ứng không thu được chất khí )
Sau khi các phản ứng xảy ra hoàn toàn thì số thí nghiệm thu được dung dịch chứa hai muối là
A. 3
B. 2
C. 4
D. 5
Tiến hành các thí nghiệm sau : (a) Cho dung dịch chưa 4a mol HCl vào dung dịch chứa a mol NaAlO2 (b) Cho Al2O3 dư vào lượng dư dung dịch NaOH (c) Sục khí CO2 đến dư vào dung dịch Ba(OH)2 (d) Cho Fe vào dung dịch Fe2(SO4)3 dư (e) Cho dung dịch chứa a mol KHSO4 vào dung dịch chứa a mol NaHCO3 (g) Cho Mg dư vào dung dịch HNO3 ( phản ứng không thu được chất khí ) Sau khi các phản ứng xảy ra hoàn toàn thì số thí nghiệm thu được dung dịch chứa hai muối là A. 3 B. 2 C. 4 D. 5
Đọc tiếp
Tiến hành các thí nghiệm sau :
(a) Cho dung dịch chưa 4a mol HCl vào dung dịch chứa a mol NaAlO2
(b) Cho Al2O3 dư vào lượng dư dung dịch NaOH
(c) Sục khí CO2 đến dư vào dung dịch Ba(OH)2
(d) Cho Fe vào dung dịch Fe2(SO4)3 dư
(e) Cho dung dịch chứa a mol KHSO4 vào dung dịch chứa a mol NaHCO3
(g) Cho Mg dư vào dung dịch HNO3 ( phản ứng không thu được chất khí )
Sau khi các phản ứng xảy ra hoàn toàn thì số thí nghiệm thu được dung dịch chứa hai muối là
A. 3
B. 2
C. 4
D. 5
X là dung dịch HCl nồng độ x mol/lít. Y là dung dịch Na2CO3 nồng độ y mol/lít. Nhỏ từ từ 100ml dung dịch X vào 100ml Y, sau các phản ứng thu được V1 lít CO2 (đktc). Nhỏ từ từ 100ml dung dịch Y vào 100ml dung dịch X, sau phản ứng thu được V2 lít CO2 (đktc). Biết tỉ lệ V1 : V2 4 : 7. Tỉ lệ x : y bằng A. 11 : 4. B. 11 : 7. C. 7 : 5. D. 7 : 3.
Đọc tiếp
X là dung dịch HCl nồng độ x mol/lít. Y là dung dịch Na2CO3 nồng độ y mol/lít. Nhỏ từ từ 100ml dung dịch X vào 100ml Y, sau các phản ứng thu được V1 lít CO2 (đktc). Nhỏ từ từ 100ml dung dịch Y vào 100ml dung dịch X, sau phản ứng thu được V2 lít CO2 (đktc). Biết tỉ lệ V1 : V2 = 4 : 7. Tỉ lệ x : y bằng
A. 11 : 4.
B. 11 : 7.
C. 7 : 5.
D. 7 : 3.
Sục từ từ CO2 vào 400 gam dung dịch Ba(OH)2. Kết quả thí nghiệm được thể hiện trên đồ thị sau: Sau khi phản ứng kết thúc, nồng độ chất tan trong dung dịch thu được là A. 42,46%. B. 64,51%. C. 50,64%. D. 70,28%.
Đọc tiếp
Sục từ từ CO2 vào 400 gam dung dịch Ba(OH)2. Kết quả thí nghiệm được thể hiện trên đồ thị sau:
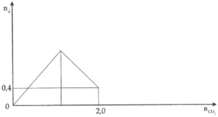
Sau khi phản ứng kết thúc, nồng độ chất tan trong dung dịch thu được là
A. 42,46%.
B. 64,51%.
C. 50,64%.
D. 70,28%.
cho 16,5 gam chất a có công thức phân tử là c2h10o3n2 vào 200 gam dung dịch naoh 8%. Sau khi các phản ứng xảy ra hoàn toàn thu được dung dịch b và khí c. Tổng nồng độ phần trăm các chất có trong b gần nhất với giá trị : A. 8%. B. 9%. C. 12%. D. 11%
Đọc tiếp
cho 16,5 gam chất a có công thức phân tử là c2h10o3n2 vào 200 gam dung dịch naoh 8%. Sau khi các phản ứng xảy ra hoàn toàn thu được dung dịch b và khí c. Tổng nồng độ phần trăm các chất có trong b gần nhất với giá trị :
A. 8%.
B. 9%.
C. 12%.
D. 11%
Cho 16,5 gam chất A có công thức phân tử là C2H10O3N2 vào 200 gam dung dịch NaOH 8%. Sau khi các phản ứng xảy ra hoàn toàn thu được dung dịch B và khí C. Tổng nồng độ phần trăm các chất có trong B gần nhất với giá trị : A. 8%. B. 9%. C. 12%. D. 11%.
Đọc tiếp
Cho 16,5 gam chất A có công thức phân tử là C2H10O3N2 vào 200 gam dung dịch NaOH 8%. Sau khi các phản ứng xảy ra hoàn toàn thu được dung dịch B và khí C. Tổng nồng độ phần trăm các chất có trong B gần nhất với giá trị :
A. 8%.
B. 9%.
C. 12%.
D. 11%.
Cho 50 ml dung dịch HNO3 2M vào 100 ml dung dịch NaOH nồng độ x mol/l, sau phản ứng thu được dung dịch chỉ chứa một chất tan duy nhất. Giá trị của x là:
A. 0,5
B. 0,8
C. 1,0
D. 0,3




