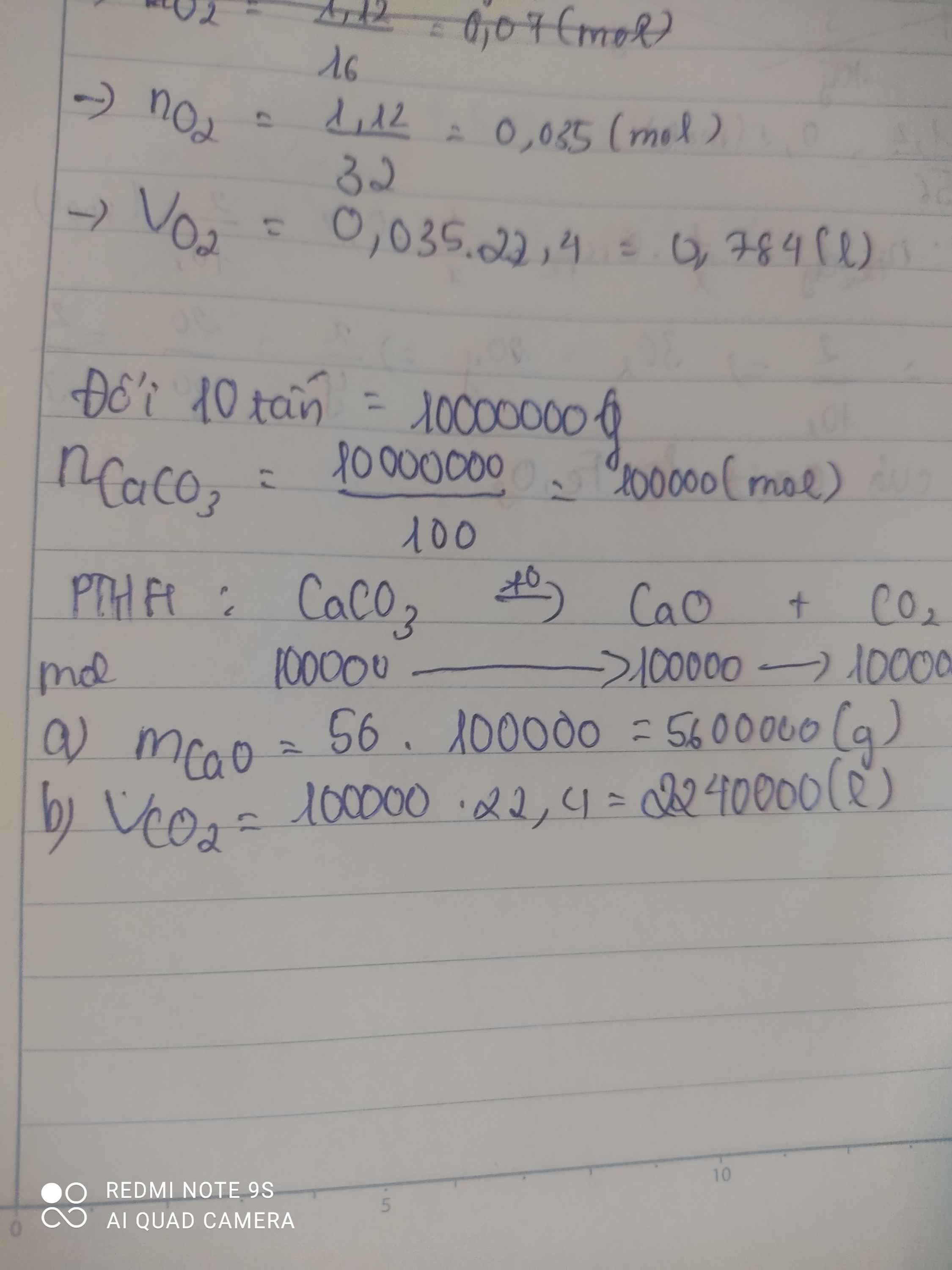PTHH : \(CaCO_3\left(t^o\right)->CaO+CO_2\uparrow\) (1)
10 tấn -> 10 000 000 g
\(n_{CaCO_3}=\dfrac{m}{M}=\dfrac{10000000}{40+12+16.3}=\text{100000}\left(mol\right)\)
Từ (1) => \(n_{CaCO_3}=n_{CaO}=\text{100000}mol\)
=> \(m_{CaO}=n.M=100000.56=5600000\left(g\right)\)
b) Từ (1) => \(n_{CaCO_3}=n_{CO_2}=\text{100000}mol\)
=> \(V_{CO_2\left(đktc\right)}=n.22,4=100000.22,4=2240000\left(l\right)\)