a)
\(n_{H_2}=\dfrac{5}{22,4}\left(mol\right)\)
PTHH: 2Na + 2H2O --> 2NaOH + H2
\(\dfrac{5}{11,2}\)<-------------------\(\dfrac{5}{22,4}\)
=> \(m_{Na}=\dfrac{5}{11,2}.23=\dfrac{575}{56}\left(g\right)\)
b)
PTHH: 2H2 + O2 --to--> 2H2O
Xét \(\dfrac{V_{O_2}}{V_{H_2}}=\dfrac{n_{O_2}}{n_{H_2}}=\dfrac{1}{2}\)
=> \(V_{O_2}=\dfrac{1}{2}.5=2,5\left(l\right)\)
\(PTHH:2Na+2H_2O\underrightarrow{t^o}2NaOH+H_2\)
0,1 0,1 0,1 0,2
\(n_{H_{2_{ }}}=\dfrac{V}{22,4}=\dfrac{5}{22,4}=0,2\left(mol\right)\)
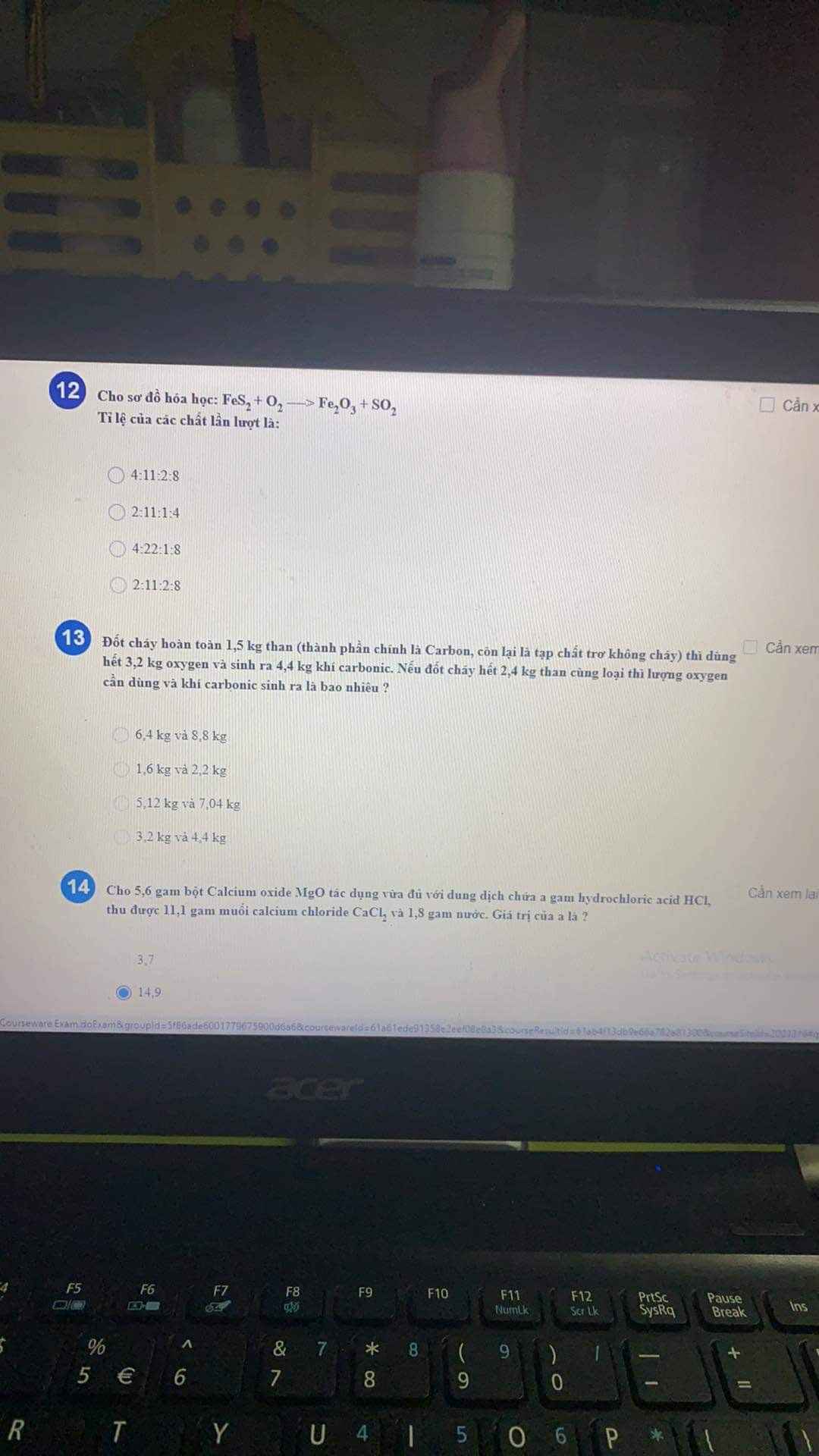
mNa=n.M=0,1.23=2,3(g)
PTHH: 2H2+O2\(\underrightarrow{t^o}\)2H2O
0,1 0,05
\(V_{O_2}\)=n.22,4=0,05.22,4=1,12l