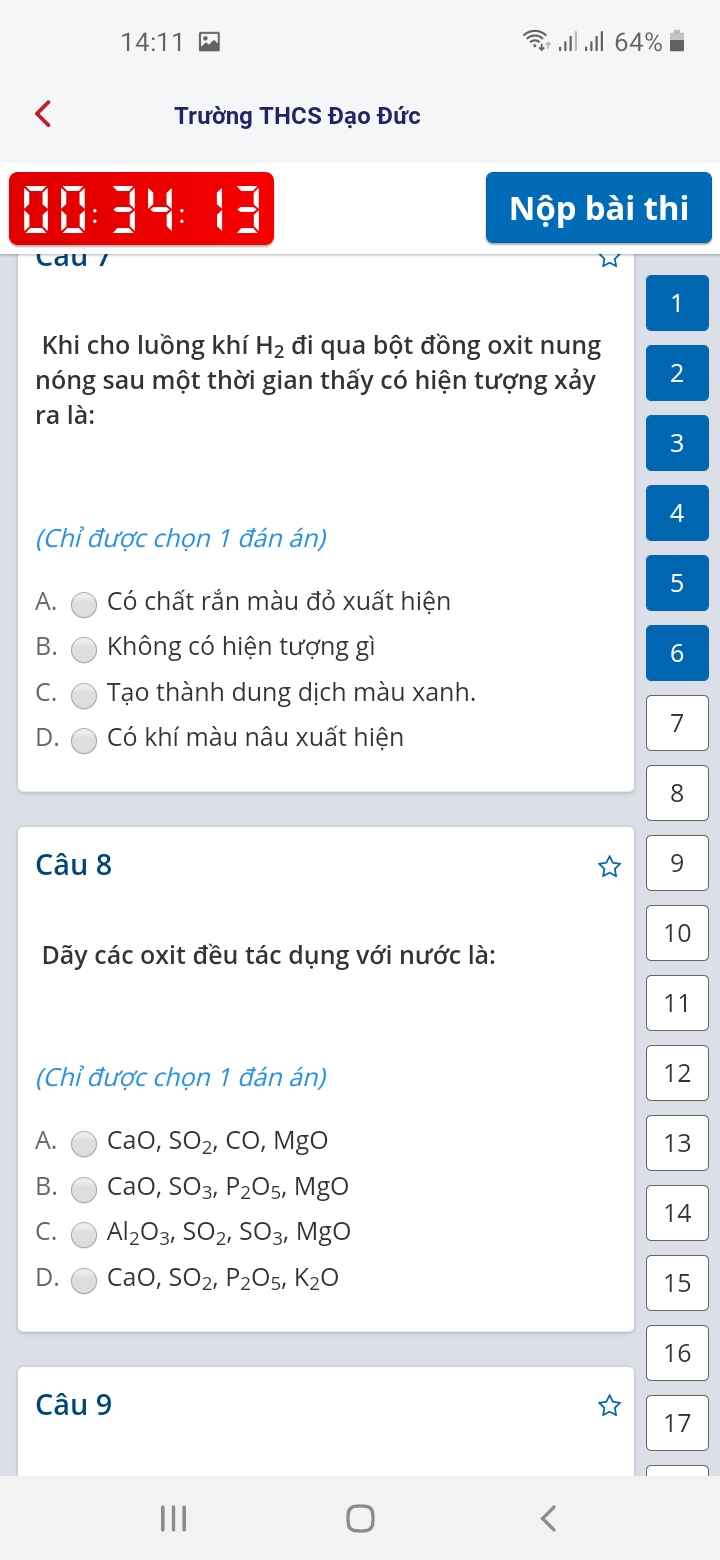
\(n_R=\dfrac{11,2}{M_R}\left(mol\right)\)
PTHH: 2R + 3Cl2 --to--> 2RCl3
\(\dfrac{11,2}{M_R}\)->\(\dfrac{16,8}{M_R}\) -->\(\dfrac{11,2}{M_R}\)
=> \(\dfrac{11,2}{M_R}.\left(M_R+106,5\right)=32,5\)
=> MR = 56 (g/mol)
=> R là Fe
\(n_{Cl_2}=\dfrac{16,8}{M_R}=\dfrac{16,8}{56}=0,3\left(mol\right)\)
=> VCl2 = 0,3.22,4 = 6,72 (l)
Đúng 2
Bình luận (0)