\(n_{CO_2}=\dfrac{3,36}{22,4}=0,15\left(mol\right)\\ a.CO_2+Ca\left(OH\right)_2\rightarrow CaCO_3+H_2O\\ b.0,15......0,15.........0,15.......0,15\left(mol\right)\\ m_{CaCO_3}=0,15.100=15\left(g\right)\)
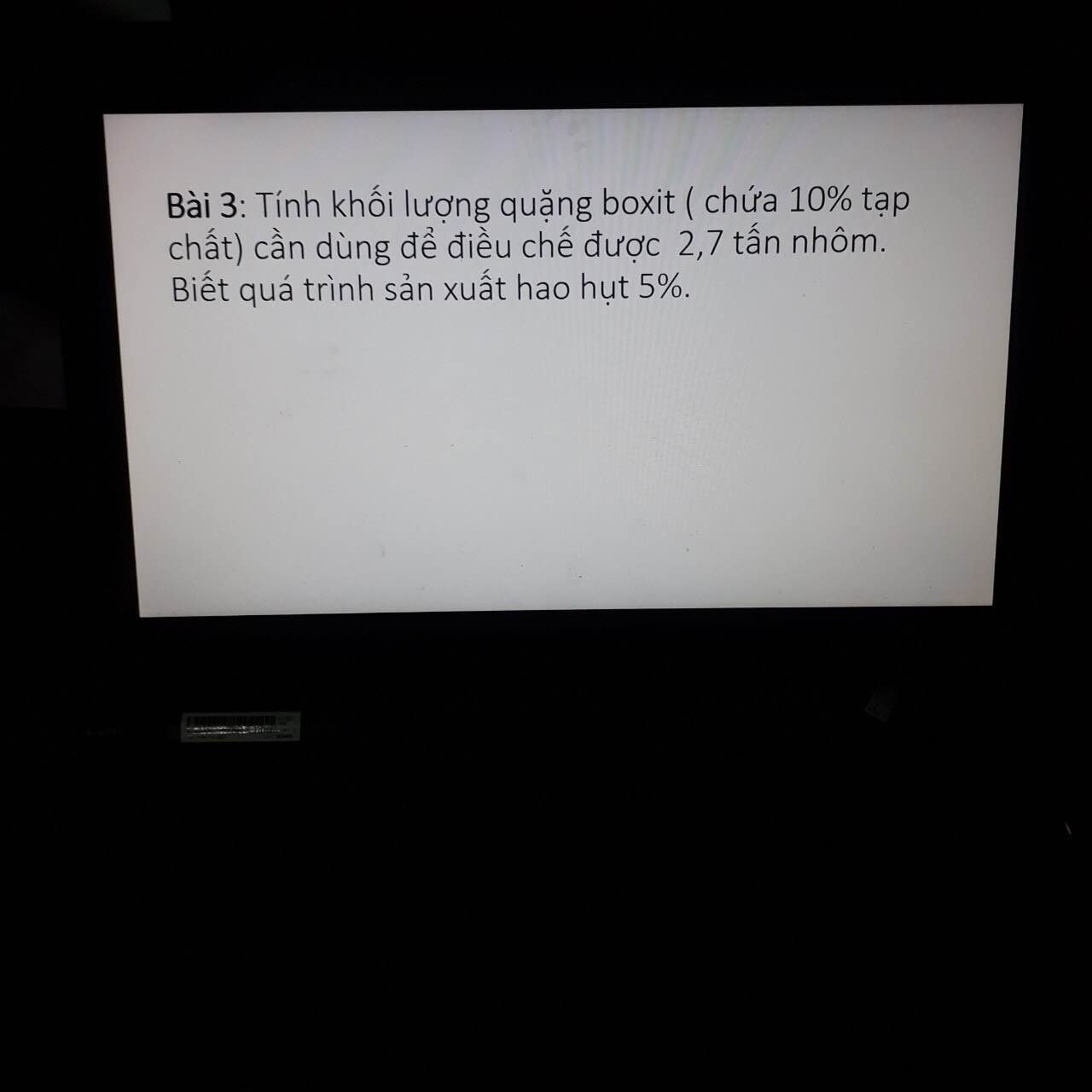
Bài 1 :
\(n_{CO2}=\dfrac{3,36}{22,4}=0,15\left(mol\right)\)
a) Pt : \(CO_2+Ca\left(OH\right)_2\rightarrow CaCO_3+H_2O|\)
1 1 1 1
0,15 0,15
b) \(n_{CaCO3}=\dfrac{0,15.1}{1}=0,15\left(mol\right)\)
⇒ \(m_{CaCO3}=0,15.100=15\left(g\right)\)
Chúc bạn học tốt