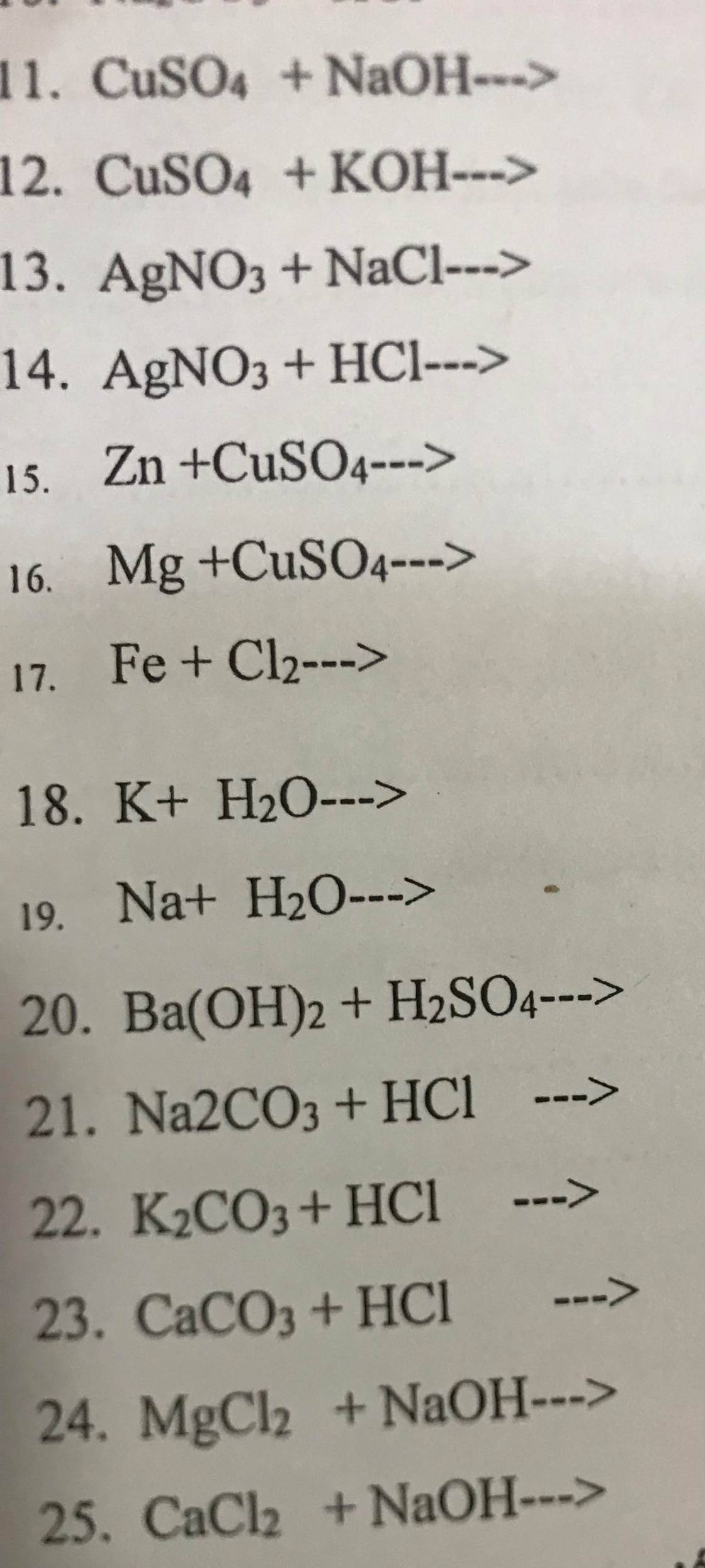\(n_{HCl}=\dfrac{3,65\%.25}{36,5}=0,025\left(mol\right)\\ Đặt:A\left(I\right)\\ 2A+2HCl\rightarrow2ACl+H_2\\ n_A=n_{HCl}=0,025\left(mol\right)\\ M_A=\dfrac{0,575}{0,025}=23\left(\dfrac{g}{mol}\right)\\ \Rightarrow A\left(I\right):Natri\left(Na=23\right)\)
=> Chọn B
Đúng 1
Bình luận (0)