\(5.MgO+2CH_3COOH\rightarrow\left(CH_3COO\right)_2Mg+2H_2O\\ MgCO_3+2CH_3COOH\rightarrow\left(CH_3COO\right)_2Mg+CO_2+H_2O\\ n_{CO_2}=0,15\left(mol\right)\\ n_{MgCO_3}=n_{CO_2}=0,15\left(mol\right)\\ \Rightarrow\%m_{MgCO_3}=\dfrac{0,15.84}{20,6}.100=61,17\%\\ \%m_{MgO}=100-61,17=38,83\%\)
Đúng 1
Bình luận (0)
Các câu hỏi tương tự
Giải hộ em hai bài này với ạ, em đang cần gấp!

Giúp mik vs ạ, mik đang cần gấp 
Mik đang cần gấp ạ
MỌI NGƯỜI GIÚP EM VỚI Ạ (EM ĐANG CẦN NÓ GẤP AÁY Ạ AI LÀM ĐƯỢC CÂU NÀO THÌ GIÚP EM CÂU ĐÓ NHÉ)
EM CẢM ƠN Ạ 
Giúp mik câu này với ạ
Hộ mk với ạ. Đang cần gấp á. Helpp me
Giúp hộ e bài 8 với ạ, e đang cần gấp ạ! E cảm ơn trước ạ 
Giúp minh bài này với ạ. Mình đang cần gấp ạ 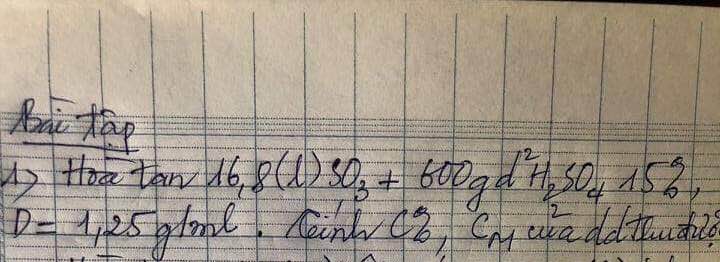
Giúp mik vs , mik đang cần gấp^^
Lm giúp mình với, mik cần gấp. Cảm ơn ạ













