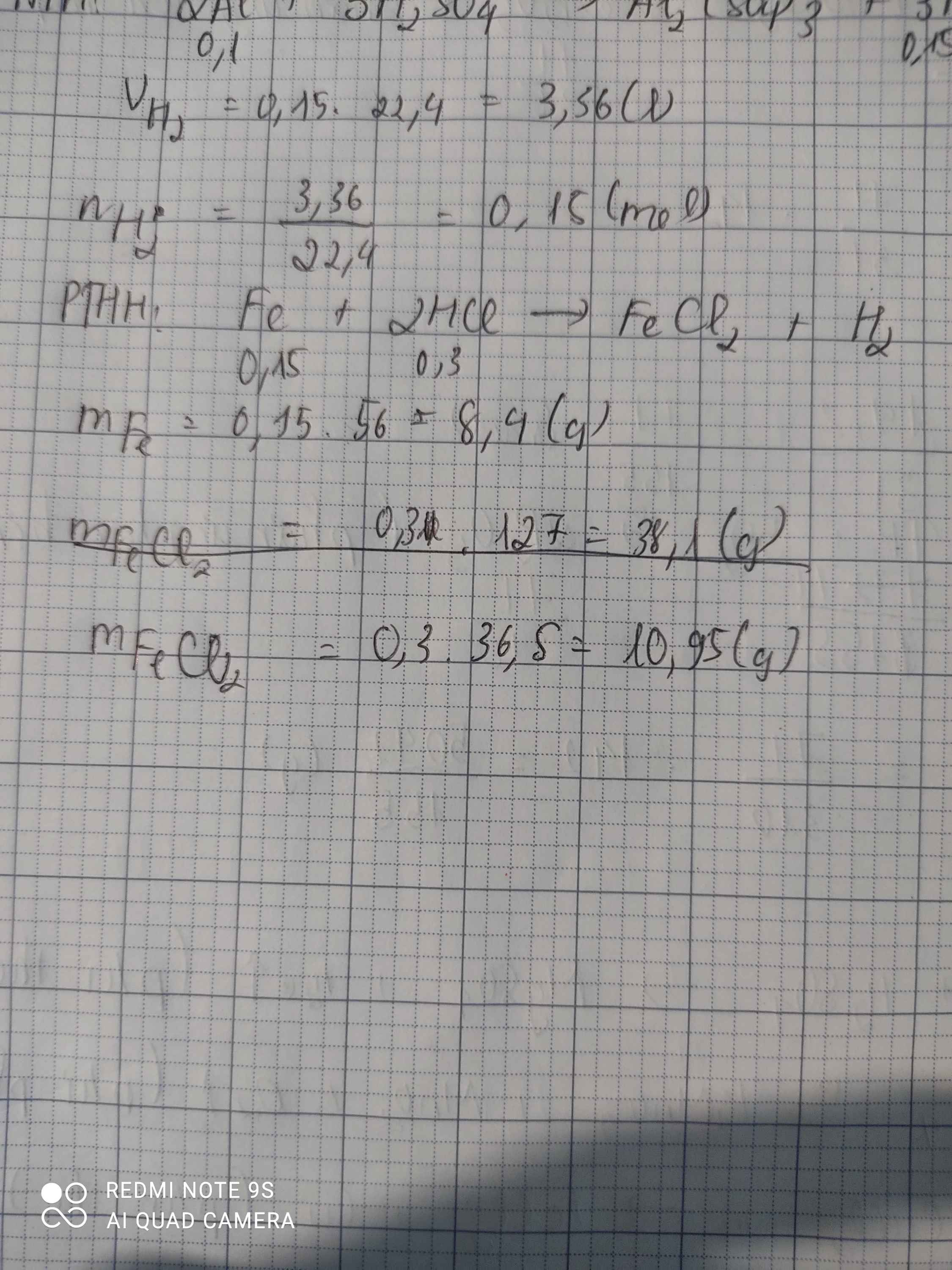a) \(n_{H_2}=\dfrac{3,36}{22,4}=0,15\left(mol\right)\)
PTHH: Fe + 2HCl --> FeCl2 + H2
0,15<-0,3<--------------0,15
=> \(m_{Fe}=0,15.56=8,4\left(g\right)\)
b) \(m_{HCl}=0,3.36,5=10,95\left(g\right)\)
a)PTHH: Fe+2HCl=>FeCl2+H2
nFe=nH2=3,36/22,4=0,15 (mol)
=>mFe=0,15x56=8,4 gam
b)mHCl tham gia phản ứng=0,15x36,5=5,475 gam
Chúc em học giỏi
a, Fe + 2HCI \(\rightarrow\) FeCI2 + H2
nFe = nH2 = 0,15 \(\rightarrow\) mFe = 8,4 g