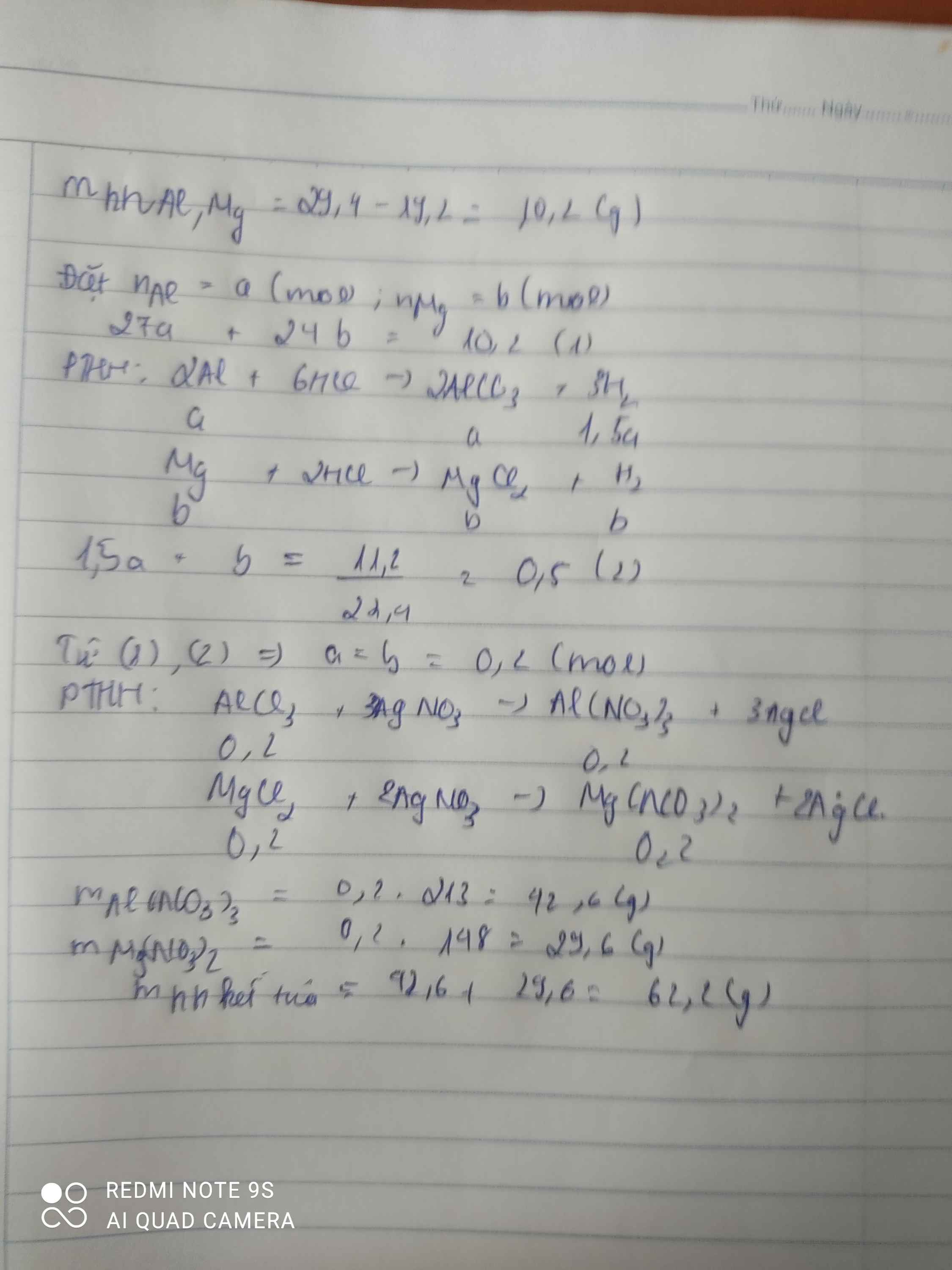n chất rắn =m Cu=19,2 g
=>m Mg, Al=29,4-19,2=10,2g
Mg+2HCl->MgCl2+H2
x-------------------------x
2Al+6HCl->2AlCl3+3H2
y--------------------------\(\dfrac{3}{2}\)y
=>Ta có :
\(\left\{{}\begin{matrix}24x+27y=10,2\\x+\dfrac{3}{2}y=\dfrac{11,2}{22,4}\end{matrix}\right.\)
=>x=0,2 mol , y=0,2 mol
=>% Cu=\(\dfrac{19,2}{29,4}\).100=65,3%
=>%Mg=\(\dfrac{0,2.24}{29,4}\).100=16,32%
=>%Al=100-65,3-16,32=18,28%
b)MgCl2+2AgNO3->2AgCl+Mg(NO3)2
0,2----------------------0,4
AlCl3+3AgNO3->Al(NO3)3+3AgCl
0,2-----------------------------------------0,6
=>m AgCl=(0,6+0,4).143,5=143,5g