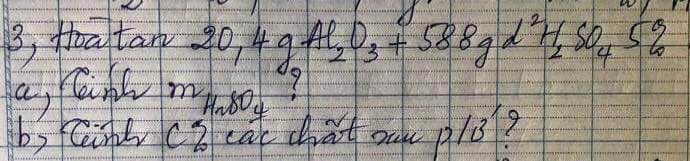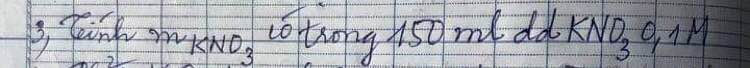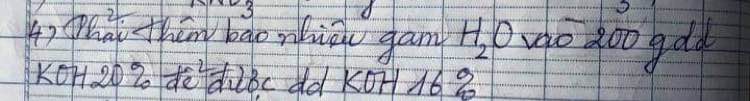Câu 2:
PTHH: \(CO_2+Ca\left(OH\right)_2\rightarrow CaCO_3\downarrow+H_2O\)
Ta có: \(n_{CO_2}=\dfrac{2,24}{22,4}=0,1\left(mol\right)=n_{Ca\left(OH\right)_2}=n_{CaCO_3}\)
\(\Rightarrow\left\{{}\begin{matrix}C_{M_{Ca\left(OH\right)_2}}=\dfrac{0,1}{0,2}=0,5\left(M\right)\\m_{CaCO_3}=0,1\cdot100=10\left(g\right)\end{matrix}\right.\)
Câu 3:
PTHH: \(Zn+H_2SO_4\rightarrow ZnSO_4+H_2\uparrow\)
Ta có: \(\left\{{}\begin{matrix}n_{Zn}=\dfrac{6,5}{65}=0,1\left(mol\right)\\n_{H_2SO_4}=\dfrac{200\cdot14,7\%}{98}=0,3\left(mol\right)\end{matrix}\right.\) \(\Rightarrow\) Axit còn dư
\(\Rightarrow\left\{{}\begin{matrix}n_{ZnSO_4}=n_{H_2}=0,1\left(mol\right)\\n_{H_2SO_4\left(dư\right)}=0,2\left(mol\right)\end{matrix}\right.\) \(\Rightarrow\left\{{}\begin{matrix}V_{H_2}=0,1\cdot22,4=2,24\left(l\right)\\m_{ZnSO_4}=0,1\cdot161=16,1\left(g\right)\\m_{H_2SO_4\left(dư\right)}=0,2\cdot98=19,6\left(g\right)\\m_{H_2}=0,1\cdot2=0,2\left(g\right)\end{matrix}\right.\)
Mặt khác: \(m_{dd}=m_{Zn}+m_{ddH_2SO_4}-m_{H_2}=206,3\left(g\right)\)
\(\Rightarrow\left\{{}\begin{matrix}C\%_{ZnSO_4}=\dfrac{16,1}{206,3}\cdot100\%\approx7,8\%\\C\%_{H_2SO_4\left(dư\right)}=\dfrac{19,6}{206,3}\cdot100\%\approx9,5\%\end{matrix}\right.\)
Câu 2:
- Dùng quỳ tím
+) Hóa đỏ: H2SO4
+) Không đổi màu: NaCl
+) Hóa xanh: Ba(OH)2 và NaOH
- Đổ dd H2SO4 vào 2 dd còn lại
+) Xuất hiện kết tủa: Ba(OH)2
PTHH: \(Ba\left(OH\right)_2+H_2SO_4\rightarrow BaSO_4\downarrow+2H_2O\)
+) Không hiện tượng: NaOH
Câu 3:
PTHH: \(CuCl_2+2NaOH\rightarrow2NaCl+Cu\left(OH\right)_2\downarrow\)
\(Cu\left(OH\right)_2\xrightarrow[]{t^o}CuO+H_2O\)
Ta có: \(\left\{{}\begin{matrix}n_{CuCl_2}=0,2\left(mol\right)\\n_{NaOH}=\dfrac{20}{40}=0,5\left(mol\right)\end{matrix}\right.\)
Xét tỉ lệ: \(\dfrac{0,2}{1}< \dfrac{0,5}{2}\) \(\Rightarrow\) NaOH còn dư
\(\Rightarrow\left\{{}\begin{matrix}n_{NaCl}=0,4\left(mol\right)\\n_{NaOH\left(dư\right)}=0,1\left(mol\right)\\n_{CuO}=n_{Cu\left(OH\right)_2}=0,2\left(mol\right)\end{matrix}\right.\) \(\Rightarrow\left\{{}\begin{matrix}m_{NaOH\left(dư\right)}=0,1\cdot40=4\left(g\right)\\m_{NaCl}=0,4\cdot58,5=23,4\left(g\right)\\m_{CuO}=0,2\cdot80=16\left(g\right)\end{matrix}\right.\)


















 giúp mình với mình cần gấp ạ
giúp mình với mình cần gấp ạ