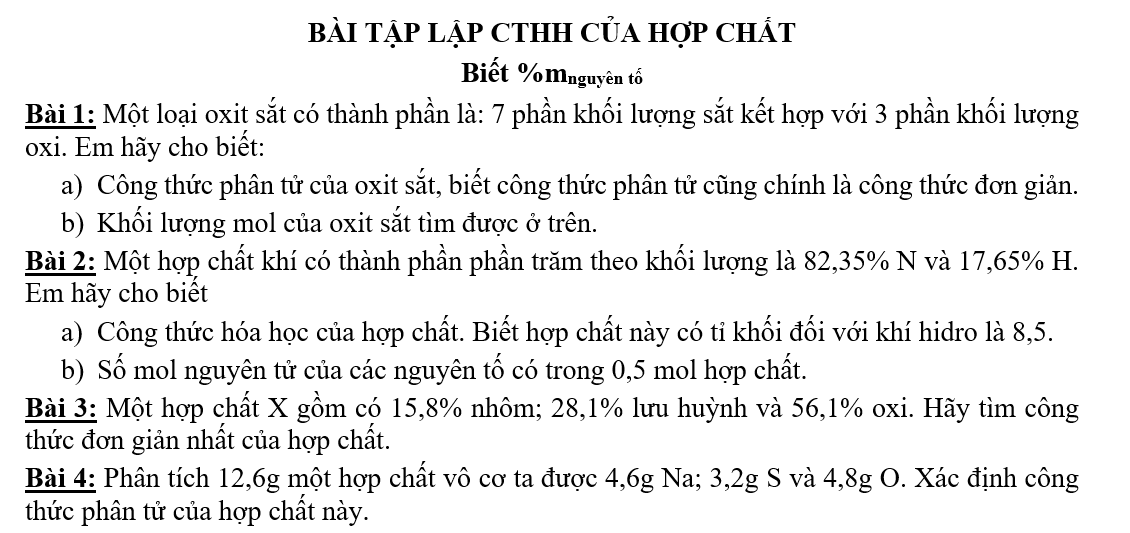
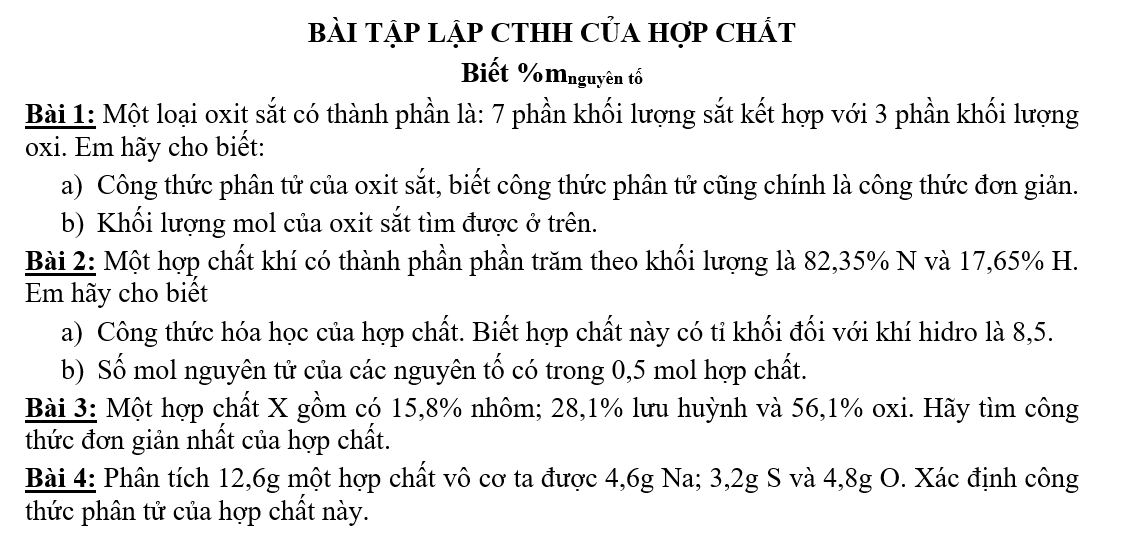
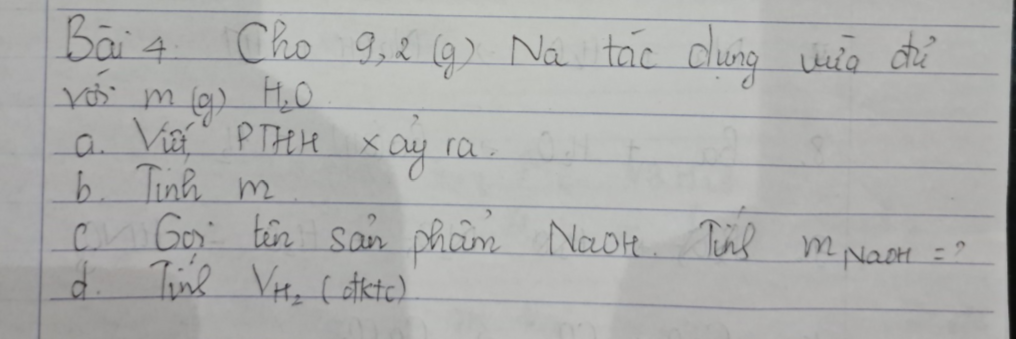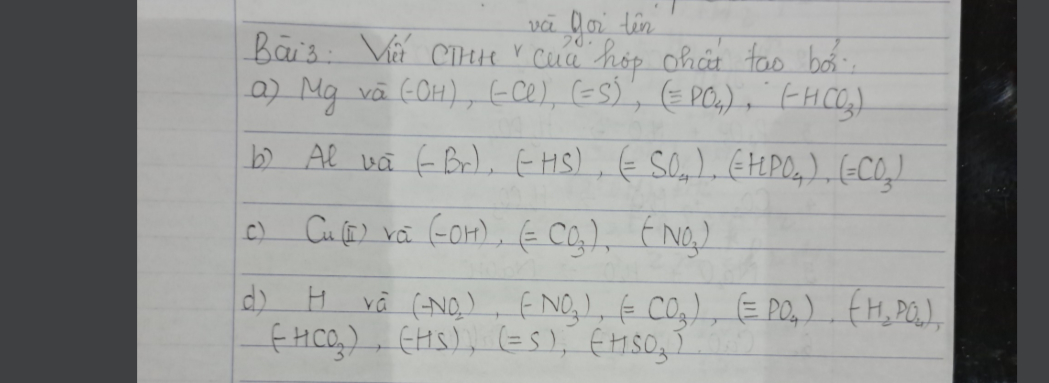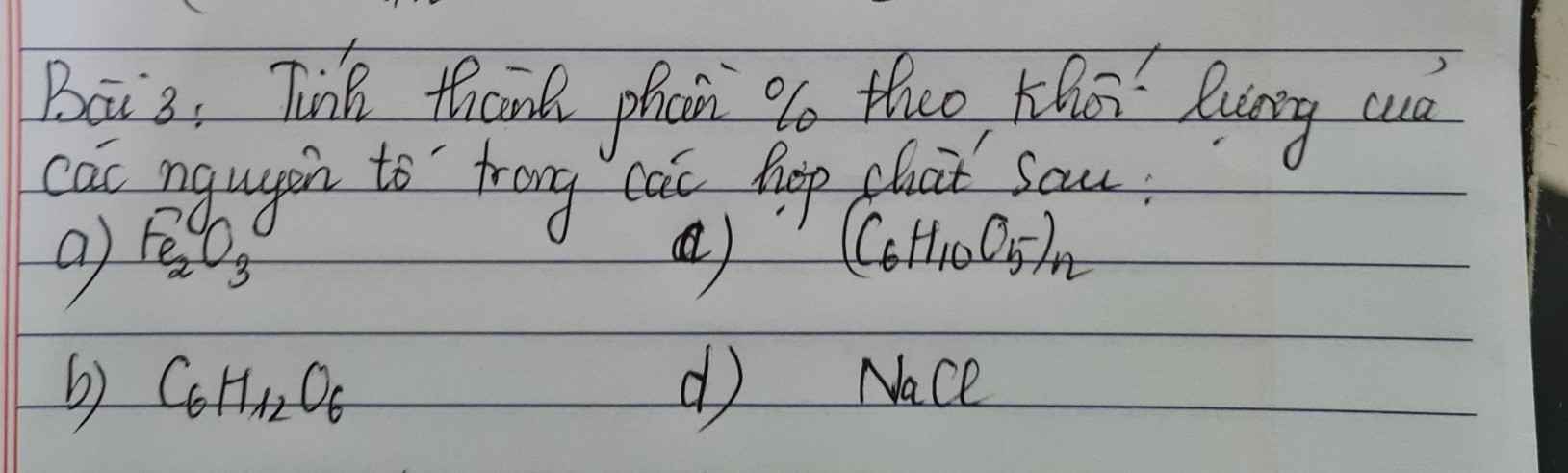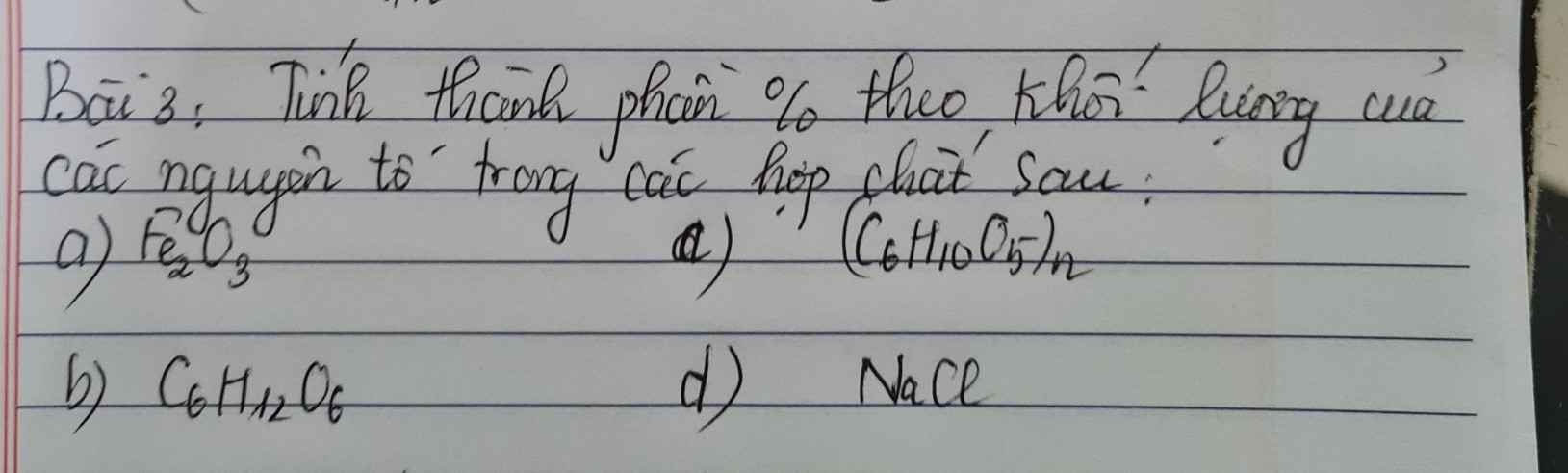Bài 4:
a. PTHH xảy ra: Na + H2O → NaOH + H2
b. nNa=\(\dfrac{m}{M}=\dfrac{9,2}{23}=0,4\)(mol)
- Theo PTHH ta có:
nNa=nH2O=nNaOH=nH2=0,4 (mol)
=> mH2O=n.M=0,4.18=7,2 (g)
c. mNaOH=n.M=0,4.40=10 (g)
d. VH2=n.24,79 (ở trường mình là 24,79)=0,4.24,79=9,916(lít)
PTHH : \(2Na+2H_2O->2NaOH+H_2\uparrow\) (1)
\(n_{Na}=\dfrac{m}{M}=\dfrac{9.2}{23}=0.4\left(mol\right)\)
Từ (1) => \(n_{Na}=n_{H_2O}=0.4\left(mol\right)\)
=> \(m=n_{H_2O}.M=0,4.\left(2+16\right)=7,2\left(g\right)\)
Từ (1) => \(n_{Na}=n_{NaOH}=0.4\left(mol\right)\)
=> \(m_{NaOH}=n.M=0,4.\left(23+16+1\right)=16\left(g\right)\)
Từ (1) => \(\dfrac{1}{2}n_{Na}=n_{H_2}=0.2\left(mol\right)\)
=> \(V_{H_2\left(đktc\right)}=n.22,4=0,2.22,4=4,48\left(l\right)\)