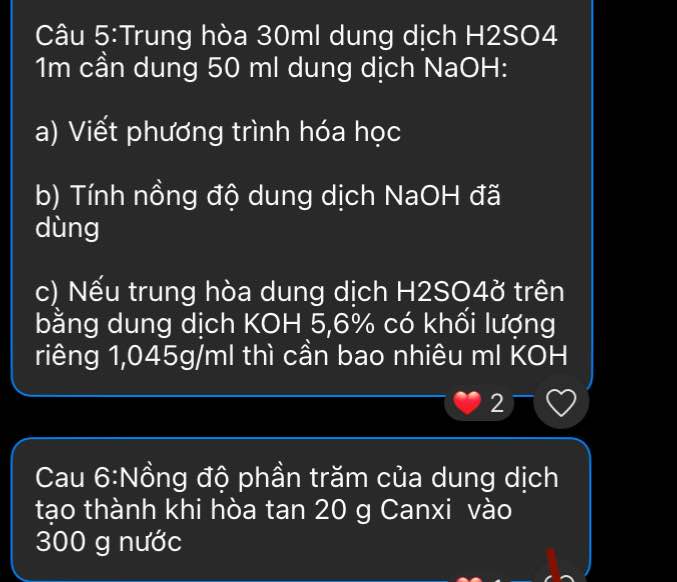
Câu 5:
a)
\(n_{H_2SO_4}=0,03.1=0,03\left(mol\right)\)
PTHH: \(2NaOH+H_2SO_4\rightarrow Na_2SO_4+2H_2O\)
0,06<--------0,03
b) \(C_{M\left(NaOH\right)}=\dfrac{0,06}{0,05}=1,2M\)
c)
PTHH: \(2KOH+H_2SO_4\rightarrow K_2SO_4+2H_2O\)
0,06<-----0,03
\(\rightarrow m_{KOH}=0,06.56=3,36\left(g\right)\\ \rightarrow m_{dd.KOH}=\dfrac{3,36}{5,6\%}=60\left(g\right)\\ \rightarrow V_{dd.KOH}=\dfrac{60}{1,045}=\dfrac{12000}{209}\left(ml\right)\)
Câu 6:
\(n_{Ca}=\dfrac{20}{40}=0,5\left(mol\right)\)
PTHH: \(Ca+2H_2O\rightarrow Ca\left(OH\right)_2+H_2\)
0,5----------------->0,5------->0,5
\(m_{dd.Ca\left(OH\right)_2}=20+300-0,5.2=319\left(g\right)\\ \rightarrow C\%_{Ca\left(OH\right)_2}=\dfrac{0,5.74}{319}.100\%=11,6\%\)