\(n_{H_2}=\dfrac{5,6}{22,4}=0,25mol\Rightarrow n_{ancol}=2n_{H_2}=0,5mol=n_{O\left(X\right)}\)
\(n_{H_2O}=\dfrac{27}{18}=1,5mol\Rightarrow m_H=1,5\cdot2=3g\)
Có \(m_X=m_C+m_H+m_O\)
\(\Rightarrow25,4=m_C+3+0,5\cdot16\)
\(\Rightarrow m_C=14,4g\Rightarrow n_C=\dfrac{14,4}{12}=1,2mol=n_{CO_2}\)
\(m_{CO_2}=1,2\cdot44=52,8g\)















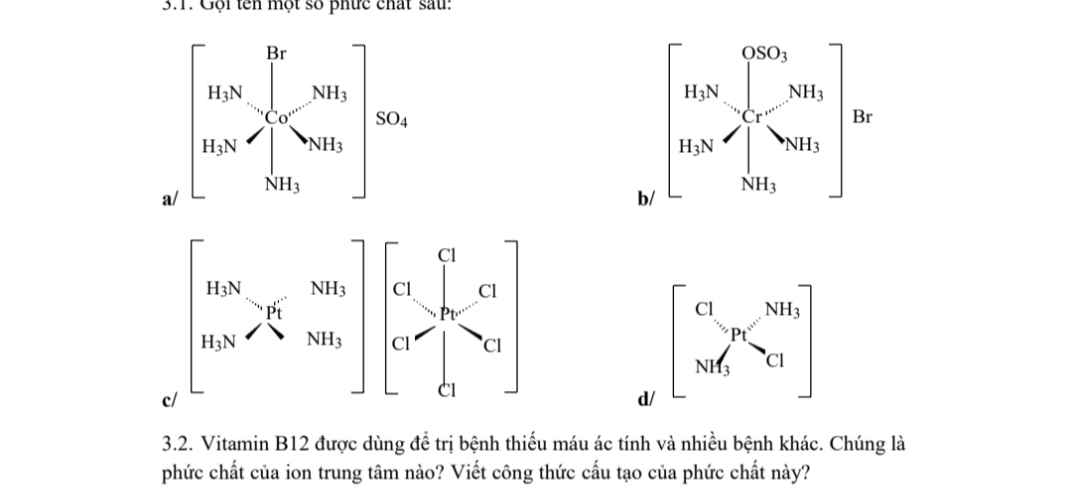




 Giúp vs ạ :
Giúp vs ạ :