PTHH: \(Fe+H_2SO_4\rightarrow FeSO_4+H_2\uparrow\)
a+b+c) Ta có: \(n_{H_2}=\dfrac{33,6}{22,4}=1,5\left(mol\right)=n_{Fe}=n_{FeSO_4}=n_{H_2SO_4}\)
\(\Rightarrow\left\{{}\begin{matrix}m_{Fe}=1,5\cdot56=84\left(g\right)\\m_{FeSO_4.5H_2O}=1,5\cdot152+1,5\cdot5\cdot18=363\left(g\right)\\C_{M_{H_2SO_4}}=\dfrac{1,5}{0,5}=3\left(M\right)\end{matrix}\right.\)
d) PTHH: \(H_2+\dfrac{1}{2}O_2\xrightarrow[]{t^o}H_2O\)
Theo PTHH: \(n_{O_2}=\dfrac{1}{2}n_{H_2}=0,75\left(mol\right)\) \(\Rightarrow n_{N_2}=0,75\cdot4=3\left(mol\right)\)
\(\Rightarrow V_{kk}=\left(0,75+3\right)\cdot22,4=84\left(l\right)\)
(Giả sử trong không khí chỉ chứa Nitơ và Oxi)











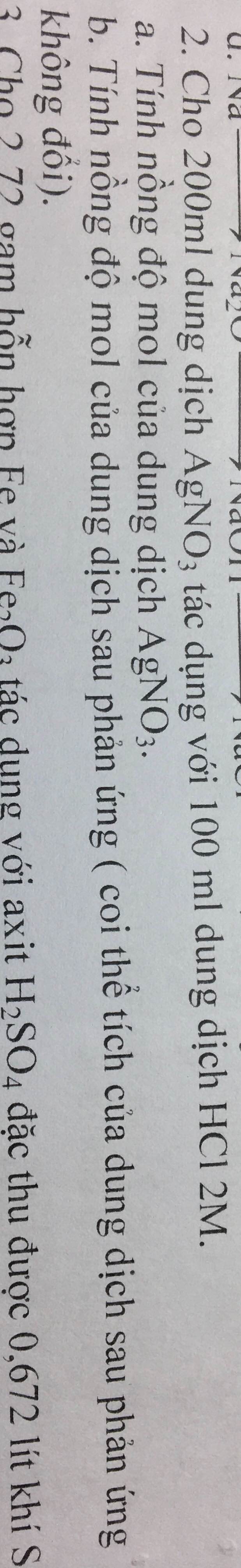

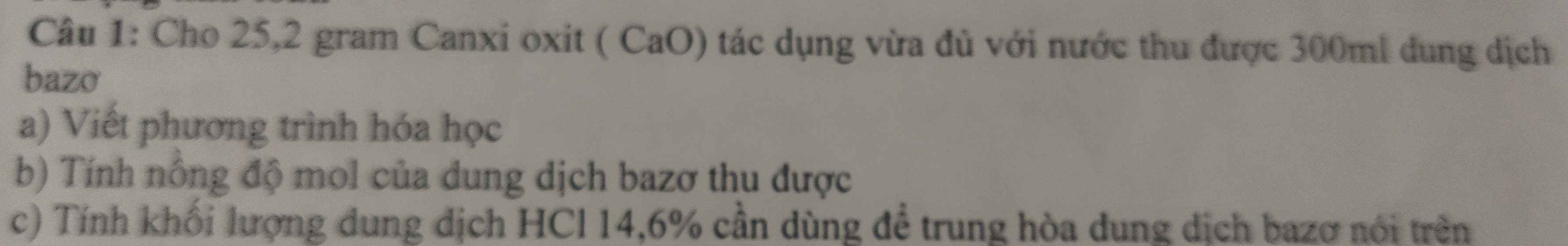
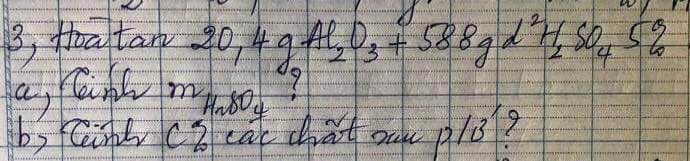





 giúp mình với mình cần gấp ạ
giúp mình với mình cần gấp ạ