3) \(n_{NaOH}=0,12.1,5=0,18\left(mol\right)\)
PTHH: NaOH + HCl ---> NaCl + H2O
0,18----->0,18--->0,18
=> \(V_{ddHCl}=\dfrac{0,18}{2}=0,09\left(l\right)\)
=> \(V_{dd.sau.pư}=0,09+0,12=0,21\left(l\right)\)
=> \(C_{M\left(NaCl\right)}=\dfrac{0,18}{0,21}=\dfrac{6}{7}M\)
PTHH: 2NaOH + H2SO4 ---> Na2SO4 + 2H2O
0,18------>0,09
=> \(m_{ddH_2SO_4}=\dfrac{0,09.98}{20\%}=44,1\left(g\right)\)
=> \(V_{ddH_2SO_4}=\dfrac{44,1}{1,25}=35,28\left(ml\right)\)











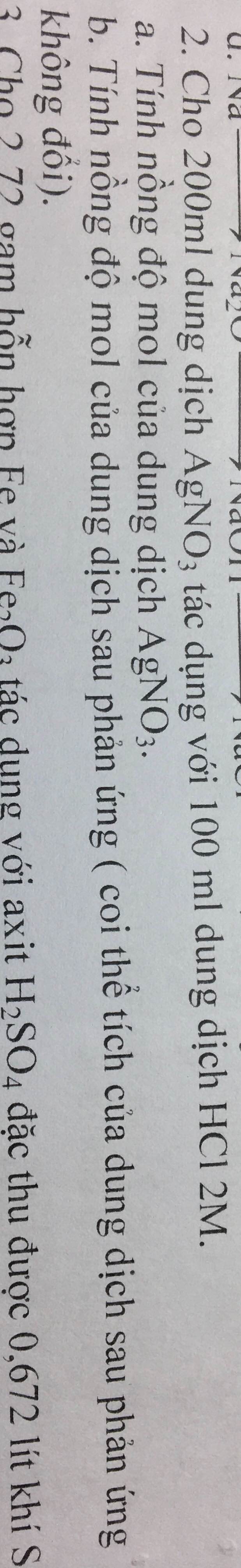

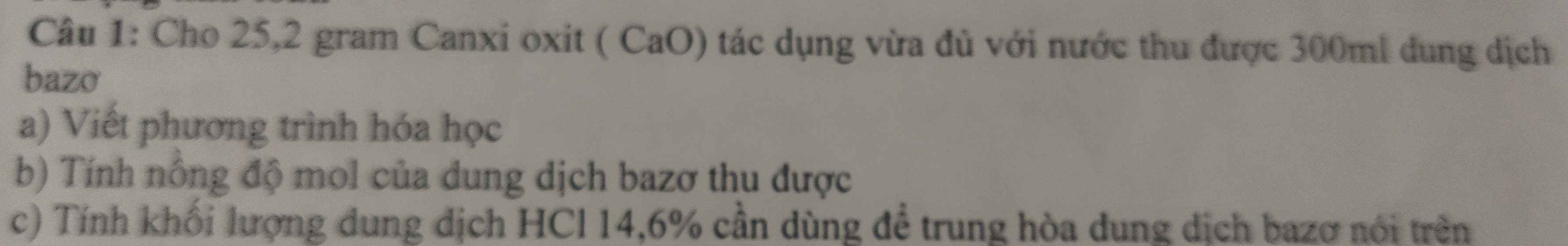
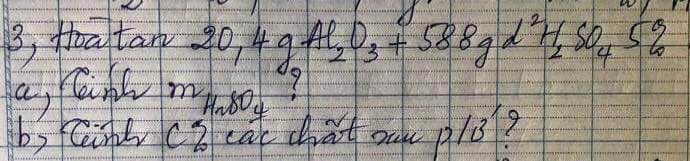





 giúp mình với mình cần gấp ạ
giúp mình với mình cần gấp ạ