\(n_{H_2SO_4}=0.06\cdot2=0.12\left(mol\right)\)
\(2NaOH+H_2SO_4\rightarrow Na_2SO_4+2H_2O\)
\(0.24...............0.12............0.12\)
\(V_{dd_{NaOH}}=\dfrac{0.24}{1}=0.24\left(l\right)\)
\(C_{M_{Na_2SO_4}}=\dfrac{0.12}{0.06+0.24}=0.4\left(M\right)\)
PTHH: H2SO4 + 2NaOH -> Na2SO4 + 2H2O(1)
0,12 -> 0,24 -> 0,12 (mol)
nH2SO4=Vdd.C(M)=0,06.2=0,12(mol)
-VddNaOH=n/C(M)=0,24/1=0,24(l)
VddSPỨ =VddH2SO4 + VddNaOH =0,06+0,24=0,3(l)
-C(M)ddA = C(M)Na2SO4=n/Vdd=0,12/0,3=0,4(M)













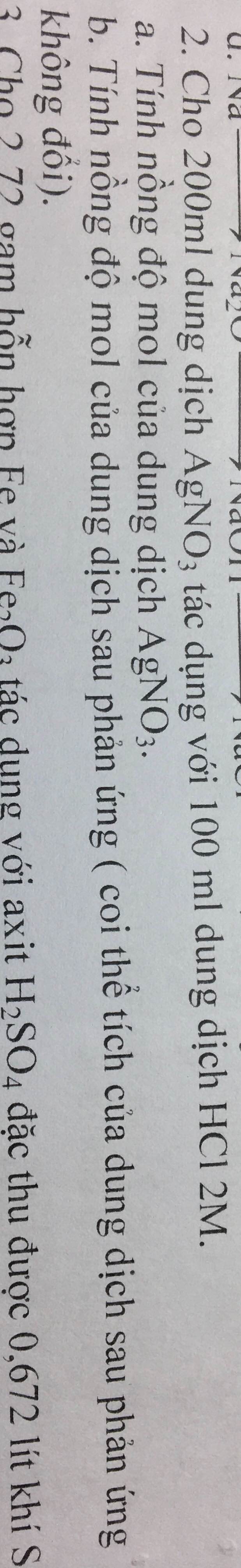

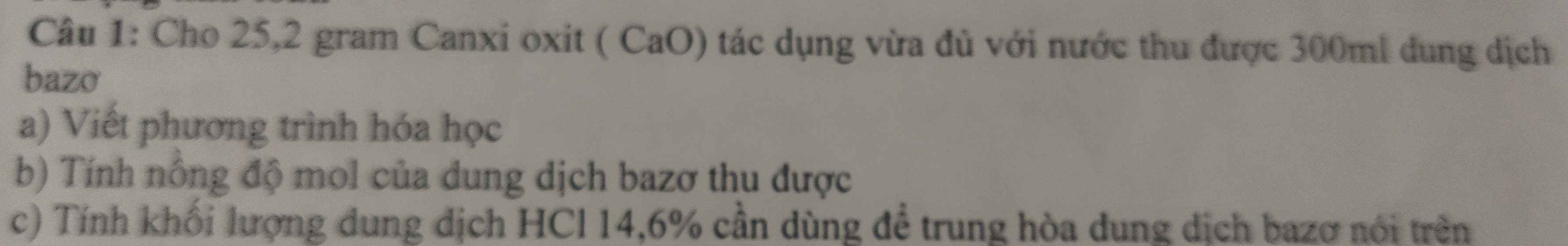
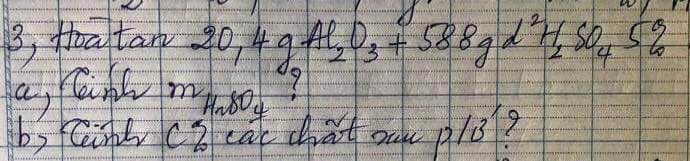





 giúp mình với mình cần gấp ạ
giúp mình với mình cần gấp ạ