Câu 2 :
\(m_{ct}=\dfrac{208.10}{100}=20,8\left(g\right)\)
\(n_{BaCl2}=\dfrac{20,8}{208}=0,1\left(mol\right)\)
a) Pt : \(BaCl_2+H_2SO_4\rightarrow BaSO_4+2HCl|\)
1 1 1 2
0,1 0,1 0,1 0,2
b) \(n_{H2SO4}=\dfrac{0,1.1}{1}=0,1\left(mol\right)\)
\(m_{H2SO4}=0,1.98=9,8\left(g\right)\)
\(m_{ddH2SO4}=\dfrac{9,8.100}{8}=122,5\left(g\right)\)
c) \(n_{BaSO4}=\dfrac{0,1.1}{1}=0,1\left(mol\right)\)
⇒ \(m_{BaSO4}=0,1.233=23,3\left(g\right)\)
d) \(n_{HCl}=\dfrac{0,1.2}{1}=0,2\left(mol\right)\)
⇒ \(m_{HCl}=0,2.36,5=7,3\left(g\right)\)
\(m_{ddspu}=208+122,5-23,3=307,2\left(g\right)\)
\(C_{HCl}=\dfrac{7,3.100}{307,2}=2,38\)0/0
Chúc bạn học tốt











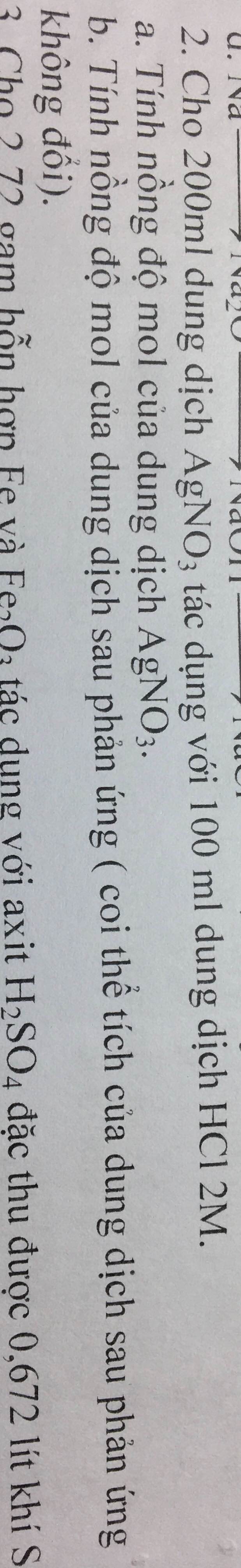

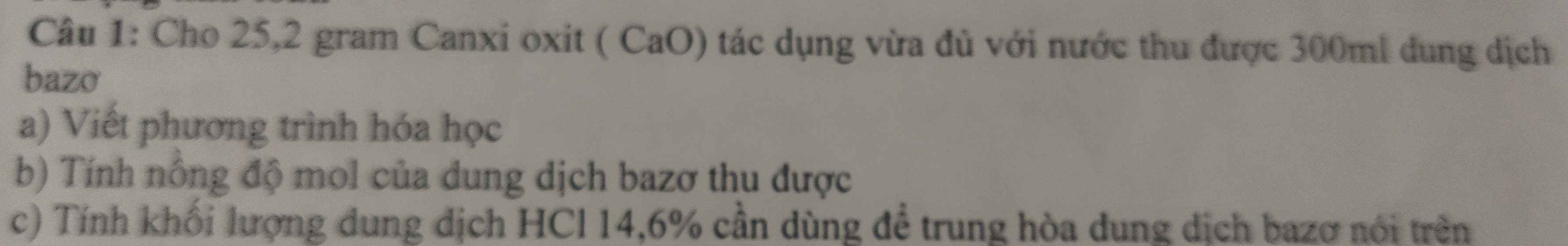
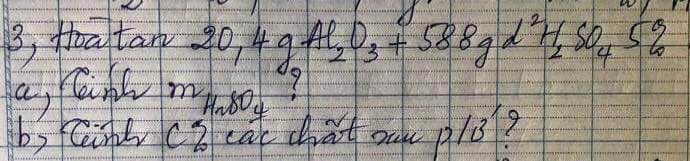





 giúp mình với mình cần gấp ạ
giúp mình với mình cần gấp ạ