\(2X+nCl_2 \xrightarrow{t^{o}} 2XCl_n\\ n_{X}=a(mol)\\ n_{XCl_n}=a(mol)\\ \frac{a.X}{a.(X+35,5n)}=\frac{1,625}{3,4}\\ \to \frac{X}{X+35,5n}=\frac{1,625}{3,4}\\ \to X=32,5n(g/mol)\\ a=2; X=65 (Zn) \)
Gọi n là hóa trị của X
PTHH: \(\dfrac{2}{n}\)X + 2HCl ---> \(\dfrac{2}{n}\)XCln + H2.
Ta có: \(n_{XCl_n}=\dfrac{3,4}{X+35,5.n}\left(mol\right)\)
\(M_{X\left(XCl_n\right)}=\dfrac{3,4}{\dfrac{3,4}{X+35,5n}}-35,5n\)
Theo PT: \(n_X=n_{XCl_n}=\dfrac{3,4}{X+35,5n}\left(mol\right)\)
=> \(M_X=\dfrac{1,625}{\dfrac{3,4}{X+35,5n}}\left(g\right)\) = \(\dfrac{3,4}{\dfrac{3,4}{X+35,5n}}-35,5n\)
Biện luận:
| n | 1 | 2 | 3 |
| X | 32,5(loại) | 65(TM) | 97,5(loại) |
Vậy X là kẽm (Zn)

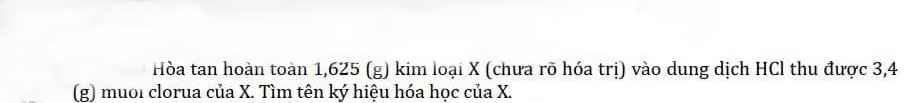









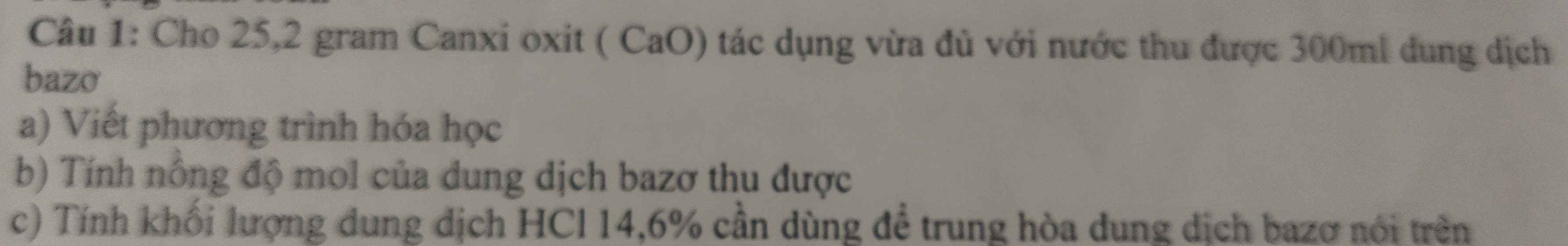






 giúp mình 2 câu này với
giúp mình 2 câu này với




