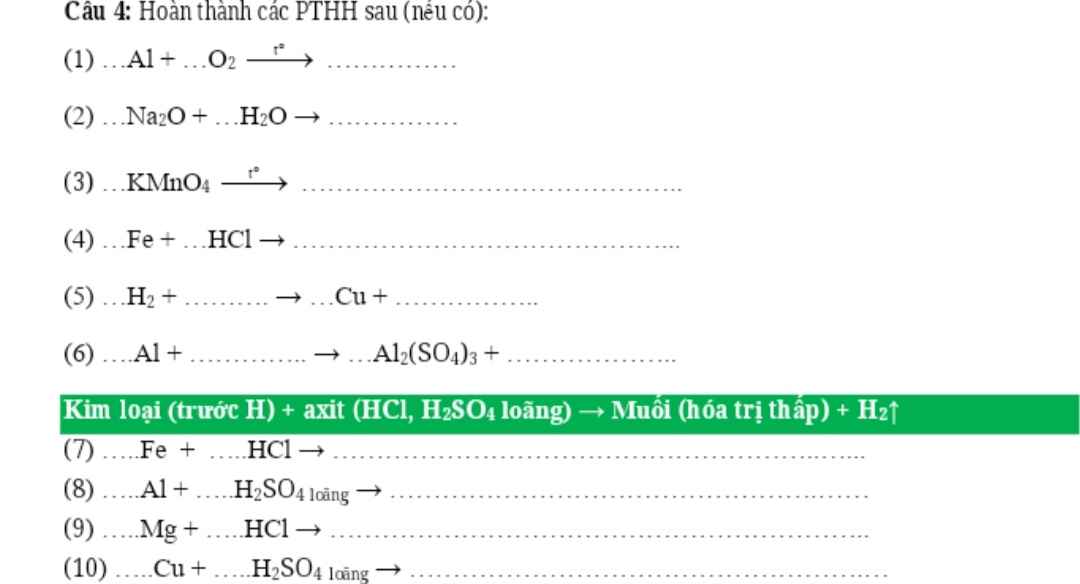
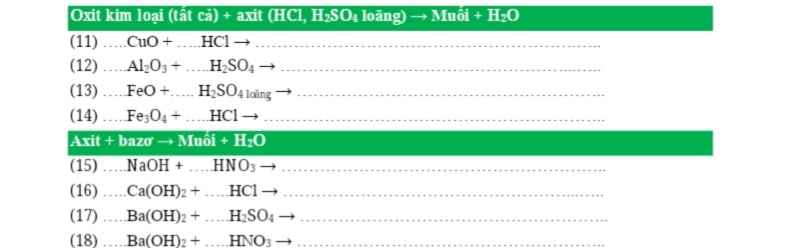
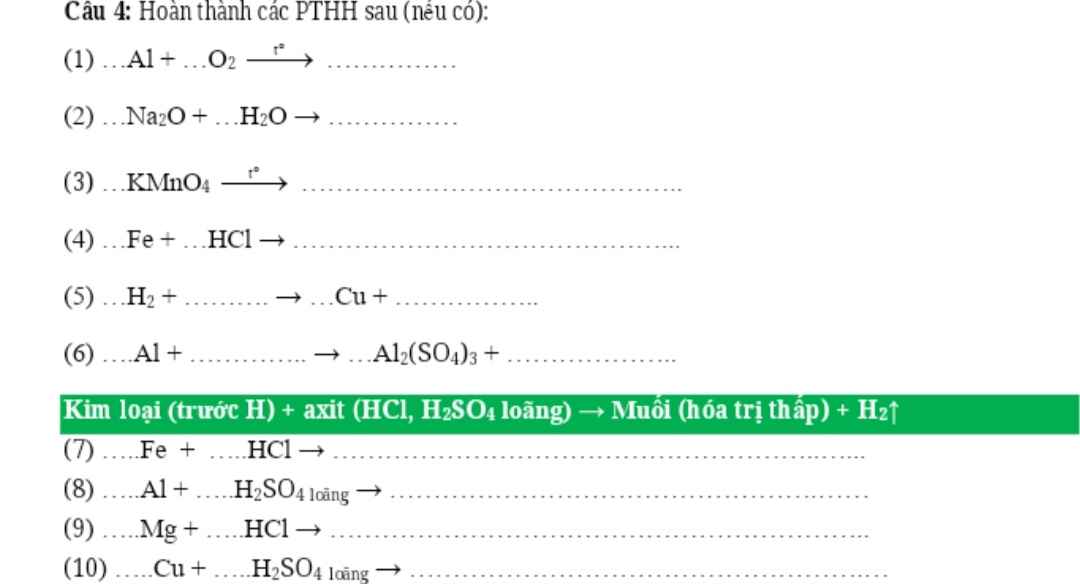
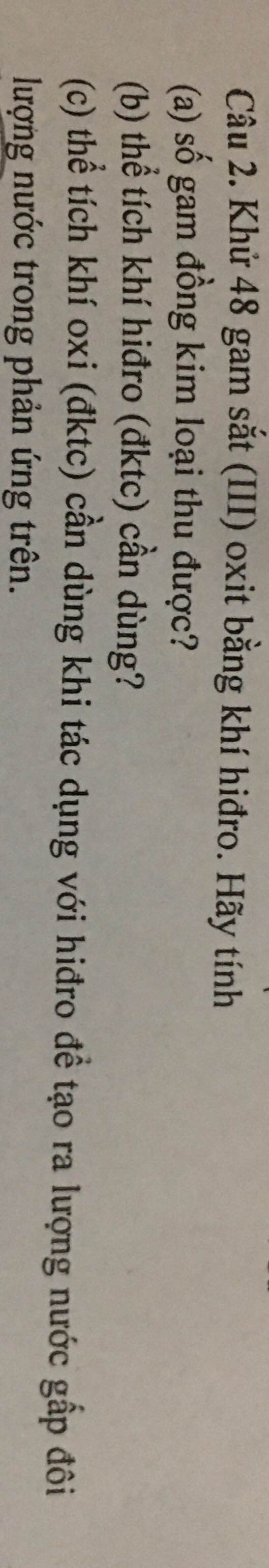nH2=3,36/22,4=0,15(mol)
a) PTHH: 2 Na + 2 H2O -> 2 NaOH + H2
x___________x_______x____0,5x(mol)
2 K + 2 H2O -> 2 KOH + H2
y____y____y______0,5y(mol)
b) Ta có hpt:
\(\left\{{}\begin{matrix}23x+39y=10,1\\0,5x+0,5y=0,15\end{matrix}\right.\Leftrightarrow\left\{{}\begin{matrix}x=0,1\\y=0,2\end{matrix}\right.\)
=> mNa= 23.0,1=2,3(g)
=>%mNa= (2,3/10,1).100=22,772%
=> %mK= 77,228%