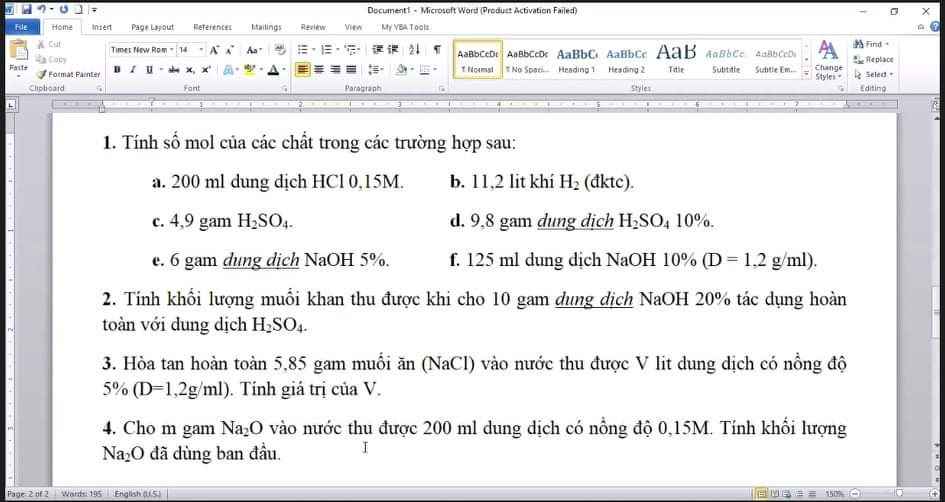
Cau 3:
Gọi số mol Mg, Zn là a, b (mol)
=> 24a + 65b = 11,3 (1)
\(n_{SO_2}=\dfrac{6,72}{22,4}=0,3\left(mol\right)\)
PTHH: Mg + 2H2SO4 --> MgSO4 + SO2 + 2H2O
a---------------------------->a
Zn + 2H2SO4 --> ZnSO4 + SO2 + 2H2O
b---------------------------->b
=> a + b = 0,3 (2)
(1)(2) => a = 0,2 (mol); b = 0,1 (mol)
=> \(\left\{{}\begin{matrix}\%m_{Mg}=\dfrac{0,2.24}{11,3}.100\%=42,5\%\\\%m_{Zn}=\dfrac{0,1.65}{11,3}.100\%=57,5\%\end{matrix}\right.\)
Câu 4:
Gọi số mol Fe, Zn là a, b (mol)
=> 56a + 65b = 18,6 (1)
\(n_{SO_2}=\dfrac{7,84}{22,4}=0,35\left(mol\right)\)
PTHH: 2Fe + 6H2SO4 --> Fe2(SO4)3 + 3SO2 + 6H2O
a-------------------------------->1,5a
Zn + 2H2SO4 --> ZnSO4 + SO2 + 2H2O
b----------------------------->b
=> 1,5a + b = 0,35 (2)
(1)(2) => a = 0,1 (mol); b = 0,2 (mol)
=> \(\left\{{}\begin{matrix}\%m_{Fe}=\dfrac{0,1.56}{18,6}.100\%=30,11\%\\\%m_{Zn}=\dfrac{0,2.65}{18,6}.100\%=69,89\%\end{matrix}\right.\)