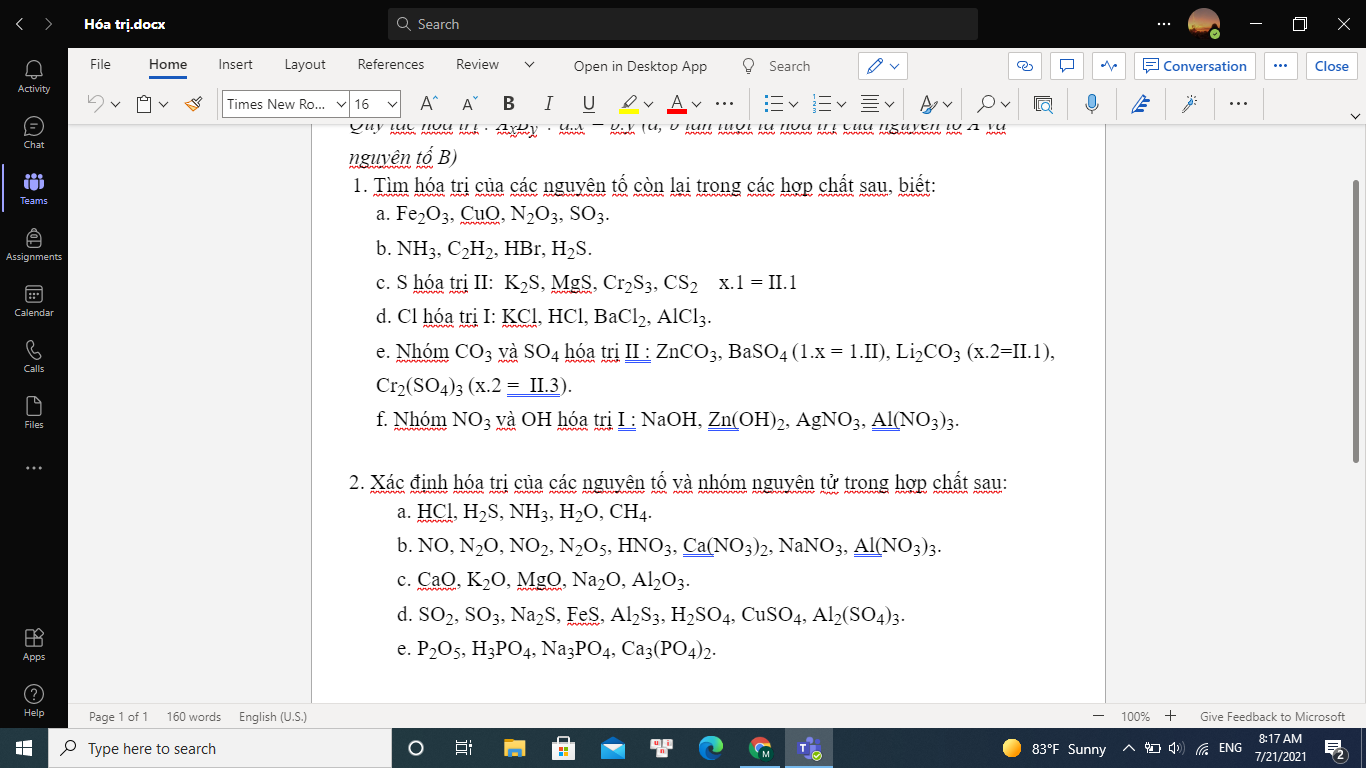
1. Vì chất rắn sau phản ứng + dd NaOH -> H2 => hh sau phản ứng chứa Al dư, Al2O3, Fe
PTHH: \(2yAl+3Fe_xO_y\xrightarrow[]{t^o}3xFe+yAl_2O_3\) (1)
Phần 1: \(Al_2O_3+6HNO_3\rightarrow2Al\left(NO_3\right)_3+3H_2O\) (2)
\(Fe+4HNO_3\rightarrow Fe\left(NO_3\right)_3+NO\uparrow+2H_2O\) (3)
\(Al+4HNO_3\rightarrow Al\left(NO_3\right)_3+NO\uparrow+2H_2O\) (4)
Phần 2: \(2Al+2NaOH+2H_2O\rightarrow2NaAlO_2+3H_2\) (5)
\(Al_2O_3+2NaOH\rightarrow2NaAlO_2+H_2O\) (6)
2. `-` Xét phần 2: \(n_{H_2}=\dfrac{0,336}{22,4}=0,015\left(mol\right)\)
Theo PT (5): \(n_{Al}=\dfrac{2}{3}n_{H_2}=0,01\left(mol\right)\)
Ta có: chất rắn không tan là Fe \(\Rightarrow n_{Fe}=\dfrac{2,52}{56}=0,045\left(mol\right)\)
Đặt \(n_{Al_2O_3}=a\left(mol\right)\)
`-` Xét phần 1:
Đặt hệ số tỉ lệ \(\dfrac{P_1}{P_2}=k\left(k>0\right)\)
`=>` \(\left\{{}\begin{matrix}n_{Al}=0,01k\left(mol\right)\\n_{Fe}=0,045k\left(mol\right)\\n_{Al_2O_3}=ak\left(mol\right)\end{matrix}\right.\)
Ta có: \(n_{NO}=\dfrac{3,696}{22,4}=0,165\left(mol\right)\)
Theo PT (3), (4): \(n_{NO}=n_{Fe}+n_{Al}\)
`=> 0,01k + 0,045k = 0,165`
`=> k = 3`
`=>` \(\left\{{}\begin{matrix}n_{Al}=0,03\left(mol\right)\\n_{Fe}=0,135\left(mol\right)\\n_{Al_2O_3}=3a\left(mol\right)\end{matrix}\right.\)
`=>` \(a=\dfrac{14,49-0,03.27-0,135.56}{102.3}=0,02\left(mol\right)\)
`-` Xét hh ban đầu:
Ta có: \(\dfrac{hhbđ}{P_2}=\dfrac{P_1+P_2}{P_2}=\dfrac{3P_2+P_2}{P_2}=4\)
`=>` \(\left\{{}\begin{matrix}n_{Al}=0,01.4=0,04\left(mol\right)\\n_{Fe}=0,045.4=0,18\left(mol\right)\\n_{Al_2O_3}=0,02.4=0,08\left(mol\right)\end{matrix}\right.\)
Theo PT (1): \(\dfrac{3x}{y}=\dfrac{n_{Fe}}{n_{Al_2O_3}}=\dfrac{0,18}{0,08}=\dfrac{9}{4}\Leftrightarrow\dfrac{x}{y}=\dfrac{3}{4}\)
Vậy CTHH của oxit sắt là Fe3O4