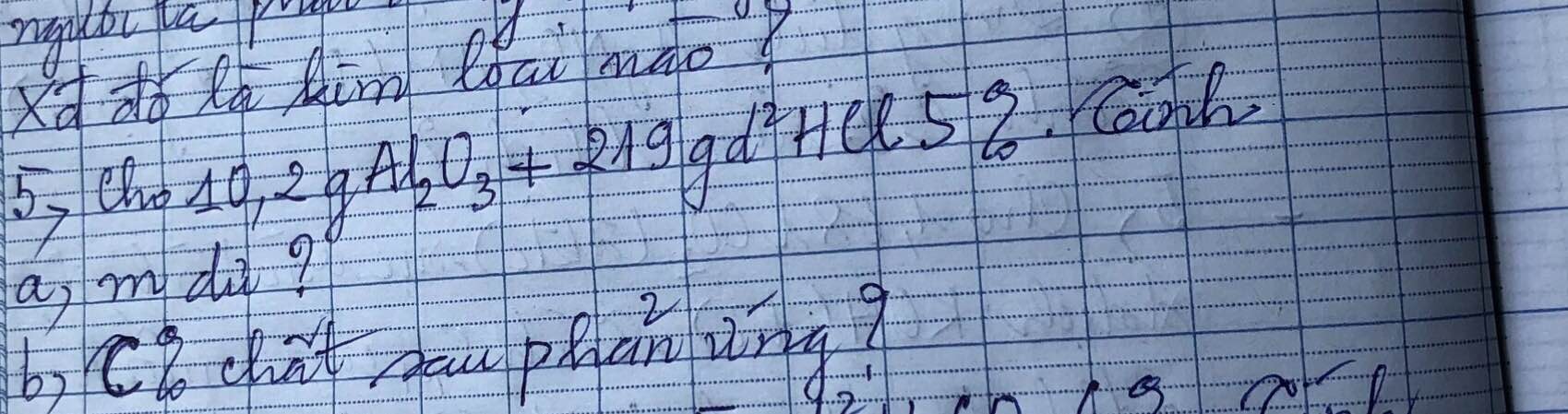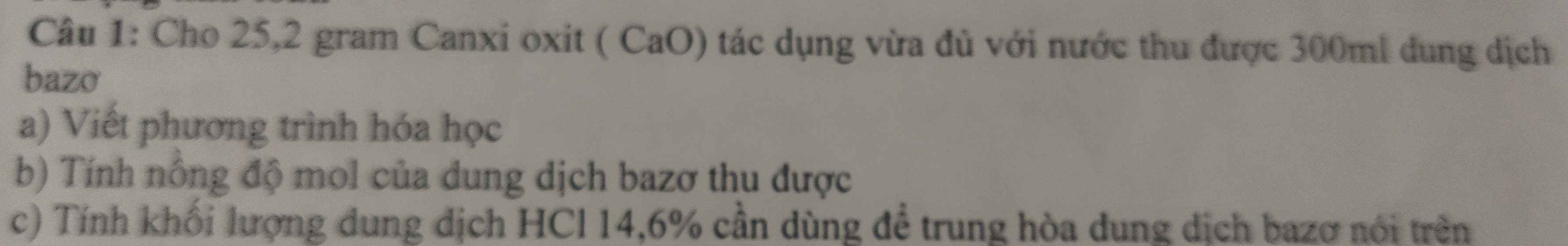a)
$n_{Al_2O_3} = \dfrac{10,2}{102} = 0,1(mol)$
$n_{HCl} = \dfrac{219.5\%}{36,5} = 0,3(mol)$
$Al_2O_3 + 6HCl \to 2AlCl_3 +3 H_2O$
$n_{Al_2O_3} : 1> n_{HCl} : 6$ nên $Al_2O_3$ dư
$n_{Al_2O_3\ pư} = \dfrac{1}{6}n_{HCl} = 0,05(mol)$
$m_{Al_2O_3\ dư} = 10,2 - 0,05.102 = 5,1(gam)$
b)
$m_{dd\ sau\ pư} = 0,05.102 + 219 = 224,1(gam)$
$n_{AlCl_3} = 2n_{Al_2O_3\ pư} = 0,1(mol)$
$C\%_{AlCl_3} = \dfrac{0,1.133,5}{224,1}.100\% = 5,96\%$
\(n_{Al_2O_3}=\frac{10,2}{102}=0,1mol\\ n_{HCl}=\frac{219.5\%}{36,5}=0,3mo\\\ Al_2O_3+6HCl \to 2 AlCl_3+3H_2O\\ Al_2O_3: 0,1 > HCl: \frac{0,3}{6}=0,05\\ \Rightarrow \text{Al2O3 du}\\ n_{Al_2O_3}=0,05mol\\ m_{Al_2O_3}=(0,1-0,05).1025,1g\ b/ C\%_{AlCl_3}=\frac{0,1.133,5}{0,05.102+219}.100=5,96\%\)