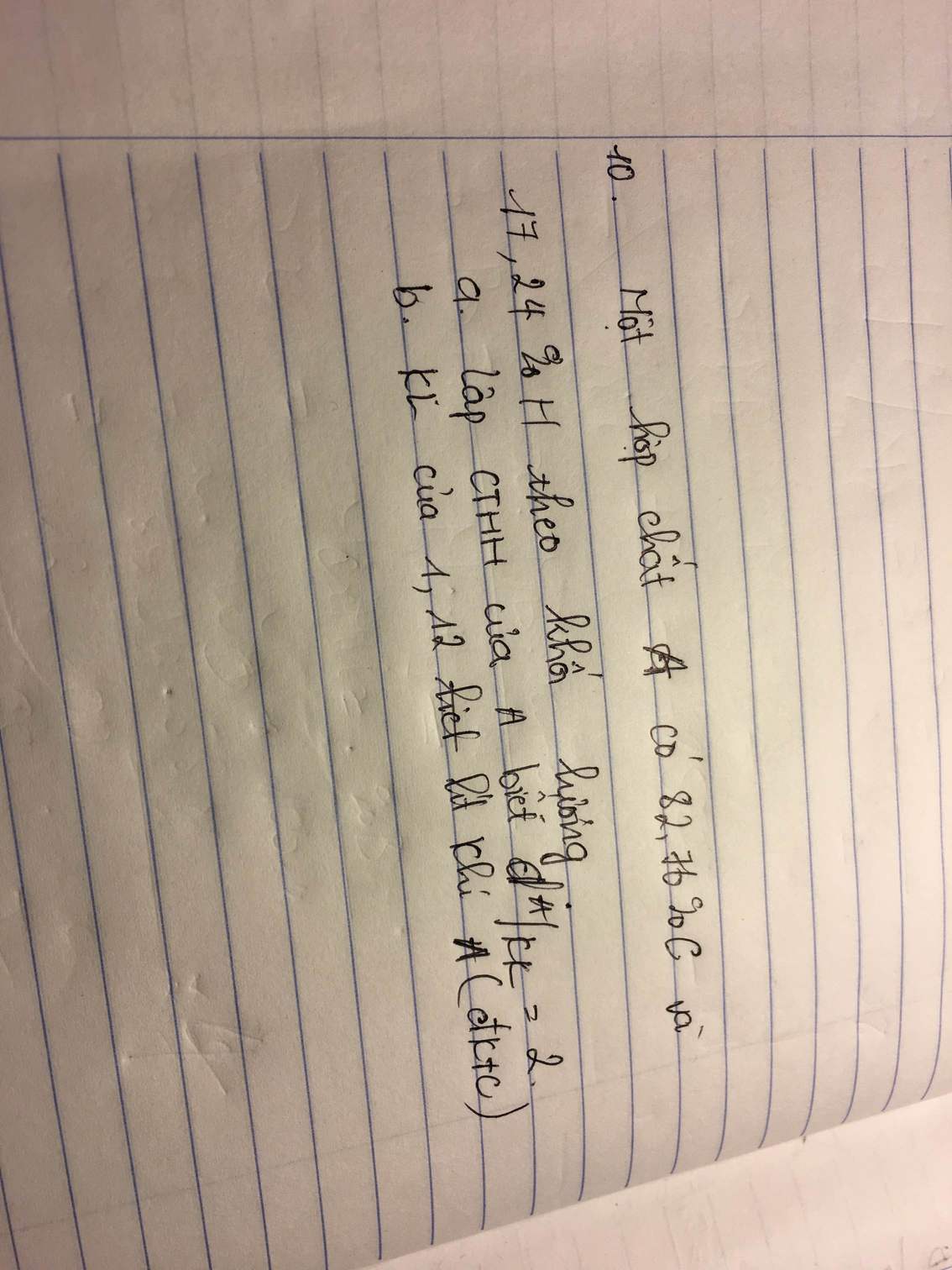a. \(d_{\dfrac{A}{kk}=2}\Rightarrow2.29=58\left(\dfrac{g}{mol}\right)\)
\(m_C=\dfrac{58.82,76\%}{100\%}=48\left(g\right)\\ n_C=\dfrac{48}{12}=4\left(mol\right)\)
\(m_H=\dfrac{58.17,24\%}{100\%}=10\left(g\right)\)
\(n_H=\dfrac{10}{1}=10\left(mol\right)\)
CTHH : C4H10
b. \(n_{C_4H_9}=\dfrac{1,12}{22,4}=0,05\left(mol\right)\\ m_{C_4H_9}=0,05.58=2,9\left(g\right)\)