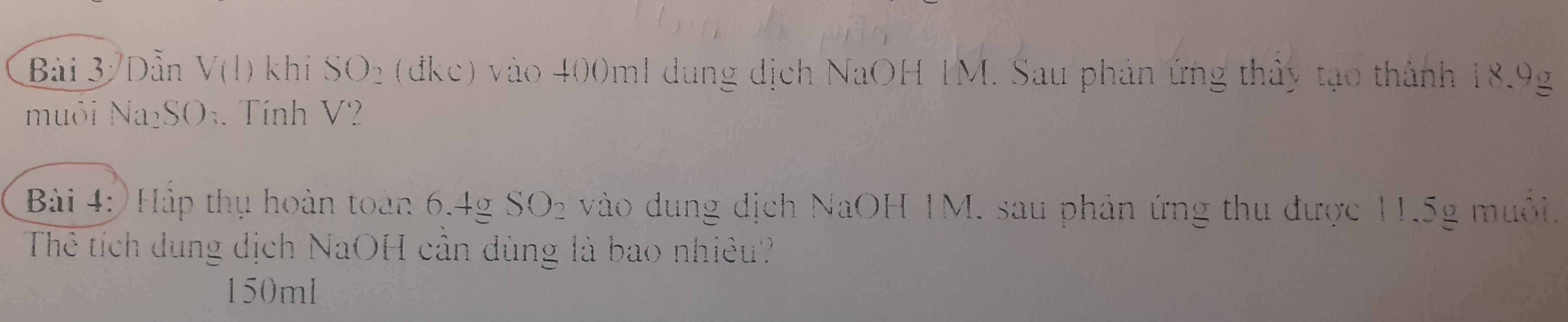Câu 16:
\(Đặt:\%n_{^{37}Cl}=a;\%n_{^{35}Cl}=100\%-a\left(a>0\right)\\ \overline{NTK}_{Cl}=35,5\\ \Leftrightarrow37.a+35.\left(100\%-a\right)=35,5\\ \Leftrightarrow a=25\%\Rightarrow\%m_{\dfrac{^{37}Cl}{MgCl_2}}=\dfrac{71}{95}.25\%.100\%=\dfrac{71}{380}\\ \Rightarrow m=m_{MgCl_2}=1,11:\dfrac{71}{380}\approx5,94\left(g\right)\)
Em ơi mình chụp rõ hơn được không em?