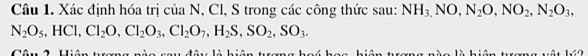\(a.PTHH:Ba+2H_2O\rightarrow Ba\left(OH\right)_2+H_2\)
\(b.n_{Ba}=\dfrac{m}{M}=\dfrac{13,7}{137}=0,1\left(mol\right)\)
\(\Rightarrow n_{H_2O}=\dfrac{1}{2}.n_{Ba}=\dfrac{1}{2}.0,1=0,05\left(mol\right)\\ \Rightarrow m_{H_2O}=n.M=0,9\left(g\right)\)
\(c.n_{Ba}=0,1\left(mol\right)\Rightarrow n_{H_2}=n_{Ba}=0,1\left(mol\right)\\ \Rightarrow V_{H_2\left(đktc\right)}=n.22,4=0,1.22,4=2,24\left(l\right)\)
Đúng 1
Bình luận (0)

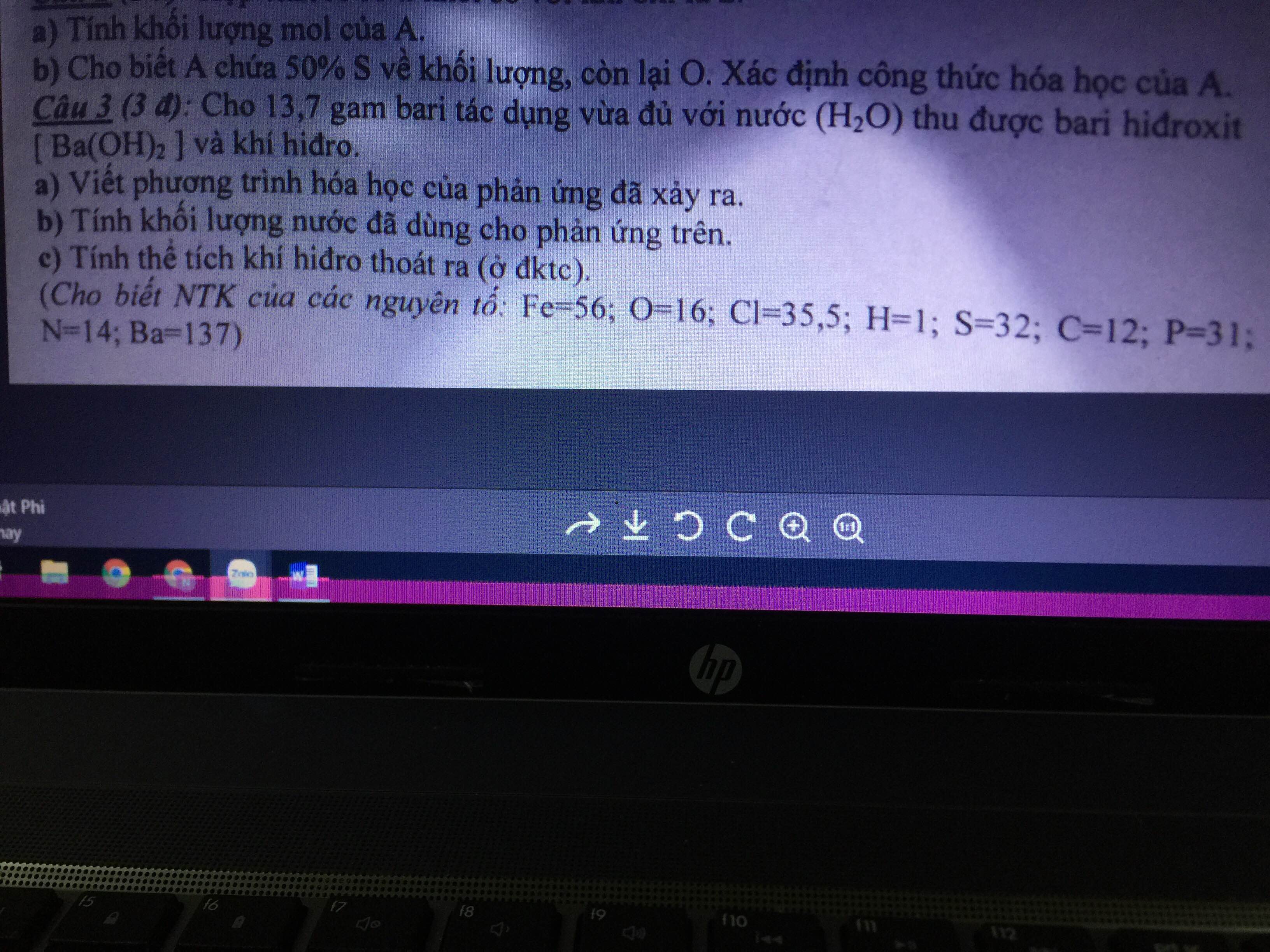









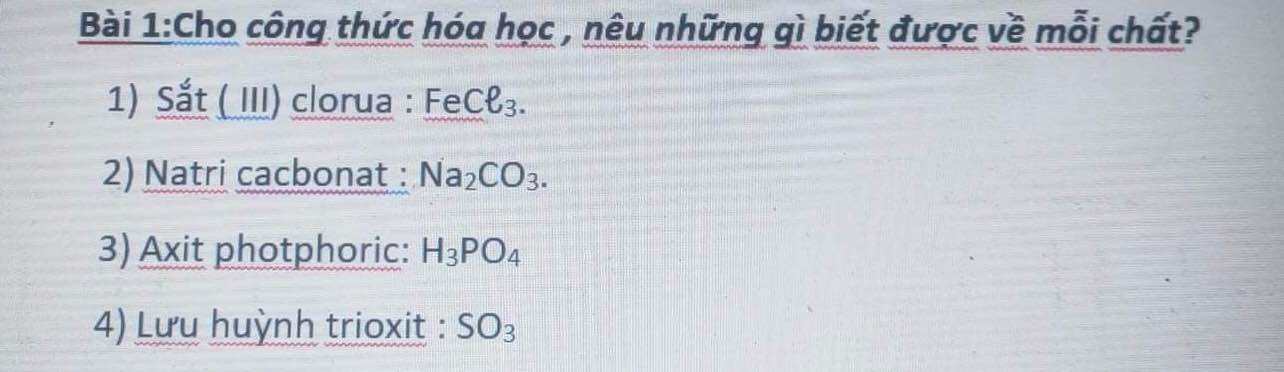




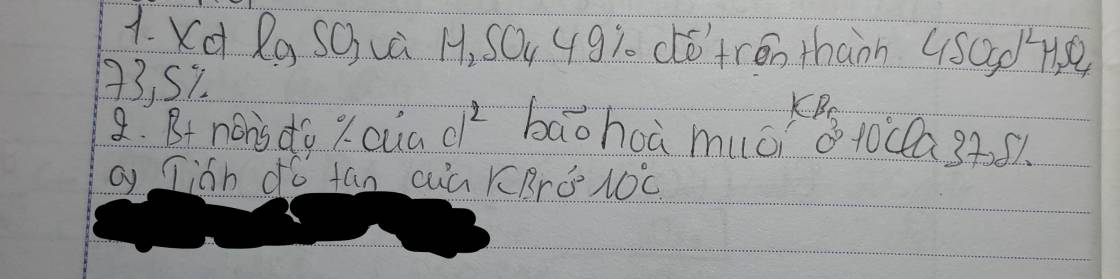 giải giúp mik vs ạ mik đang cần gấp!!!
giải giúp mik vs ạ mik đang cần gấp!!!