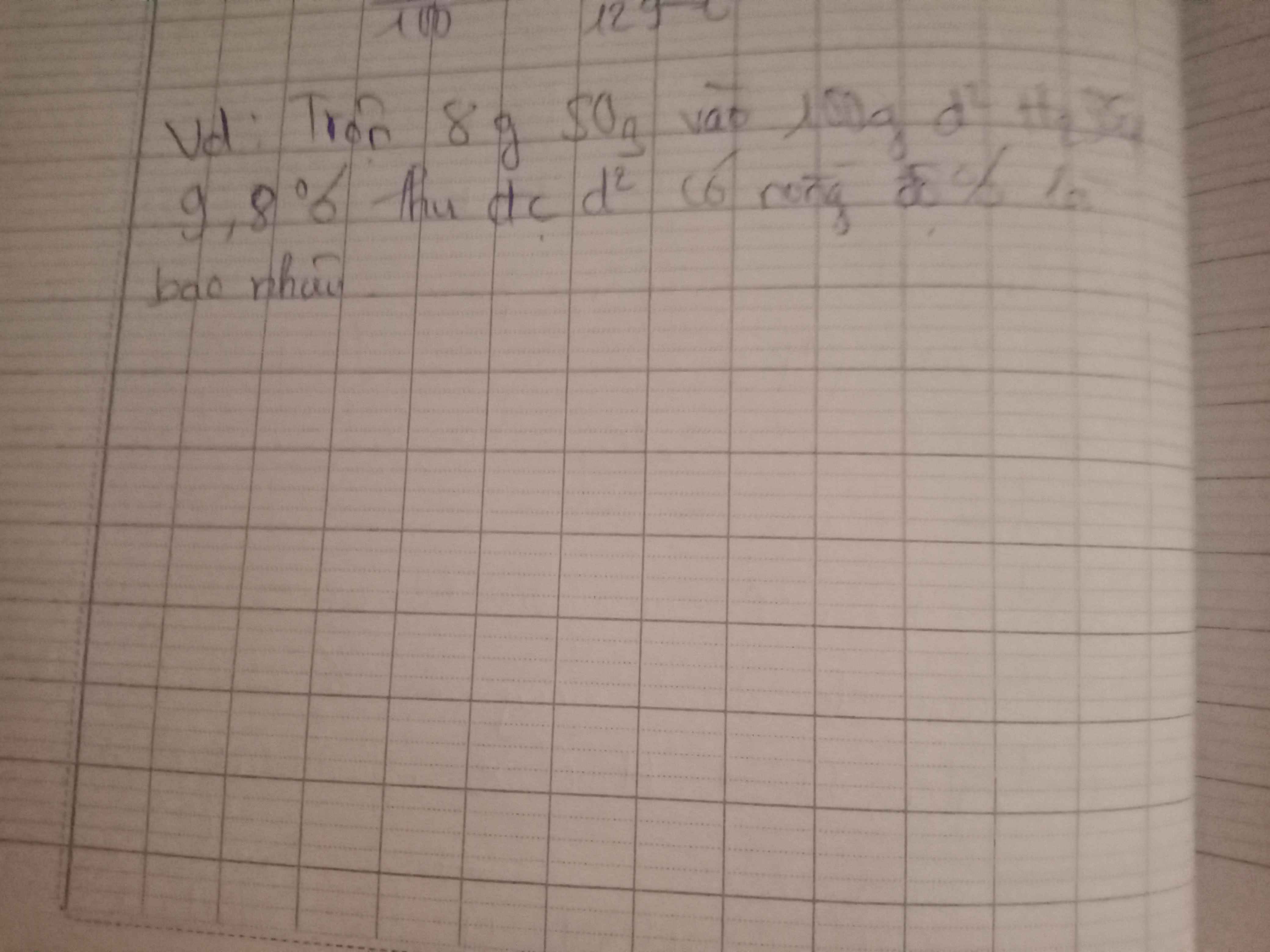
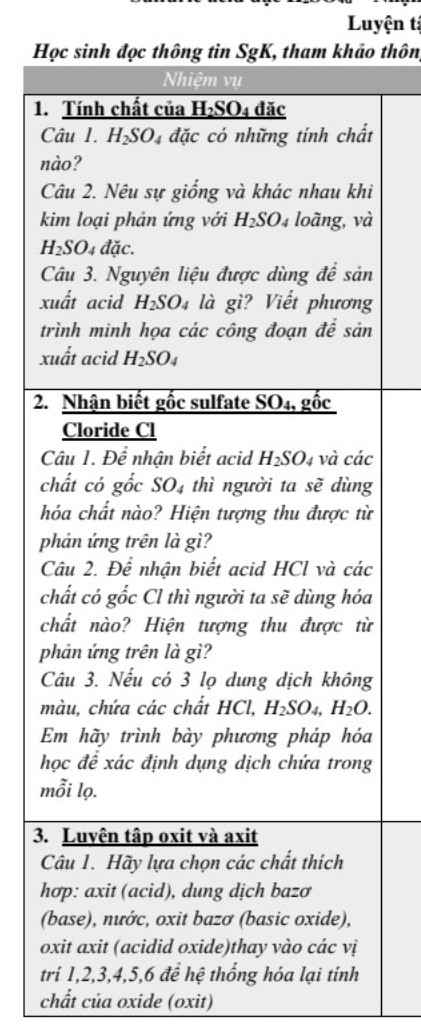
Bài 5:
\(n_{Fe_2O_3}=\dfrac{32}{160}=0,2(mol)\\ a,PTHH:Fe_2O_3+6HCl\to 2FeCl_3+3H_2O\\ b,n_{HCl}=6n_{Fe_2O_3}=1,2(mol)\\ \Rightarrow V_{dd_{HCl}}=\dfrac{1,2}{2}=0,6(l)\\ c,n_{FeCl_3}=2n_{Fe_2O_3}=0,4(mol)\\ \Rightarrow m_{FeCl_3}=0,4.162,5=65(g)\)
Câu 6:
\(n_{H_2}=\dfrac{1,12}{22,4}=0,05(mol)\\ n_{Al}=x(mol);n_{Fe}=y(mol)\\ \Rightarrow 27x+56y=1,66(1)\\ a,2Al+6HCl\to 2AlCl_3+3H_2\\ Fe+2HCl\to FeCl_2+H_2\\ \Rightarrow 1,5x+y=0,05(2)\\ (1)(2)\Rightarrow x=y=0,02(mol)\\ b,\%_{Al}=\dfrac{0,02.27}{1,66}.100\%=32,53\%\\ \%_{Fe}=100\%-32,53\%=67,47\%\)