Câu 2 :
\(1.Al_2O_3\\ 2.Fe\left(NO_3\right)_3\\ 3.CuCl_2\\ 4.SO_4\)
Câu 4 :
a) \(n_{O_2}=\dfrac{1,8.10^{23}}{6.10^{23}}=0,3\left(mol\right)\)
b) \(m_{HCl}=0,5.36,5=18,25\left(g\right)\)
c) \(n_{N_2}=\dfrac{12,395}{22,4}\approx0,6\left(mol\right)\\ m_{N_2}=0,6.28=16,8\left(g\right)\)
d) \(d\dfrac{O_2}{kk}=\dfrac{32}{29}=1,1034>1\)
=> Khí Oxi nặng hơn không khí 1,1034 lần
Câu 3 :
\(M_{Fe\left(OH\right)_3}=56+\left(16+1\right).3=107\left(DvC\right)\)
\(\%Fe=\dfrac{56}{107}.100\%=52,3\%\\ \%O=\dfrac{16.3}{107}.100\%=44,8\%\\ \%H=100\%-52,3\%-44,8\%=2,9\%\)
Câu 1:
\(a,PTHH:Zn+HCl\rightarrow ZnCl_2+H_2\)
b, Áp dụng ĐLBTKL, ta có:
\(m_{Zn}+m_{HCl}=m_{ZnCl_2}+m_{H_2}\)
\(c,\Rightarrow m_{HCl}=m_{ZnCl_2}+m_{H_2}-m_{Zn}=27,2+0,4-13=14,6\left(g\right)\)
Câu 2:
\(1,Al_2O_3\\ 2,Fe\left(NO_3\right)_3\\ 3,CuCl_2\\ 4,SO_2\)
Câu 3:
\(\%Fe=\dfrac{m_{Fe}}{M_{Fe\left(OH\right)_3}}=\dfrac{56}{107}=52,33\%\\ \%O=\dfrac{m_O}{M_{Fe\left(OH\right)_3}}=\dfrac{48}{107}=44,85\%\\ \%H=100\%-\%Fe-\%O=100\%-52,33\%-44,85\%=2,82\%\\ \\ \\ \\\)
Câu 4:
\(1,n_{O_2}=\dfrac{1,8.10^{23}}{6.10^{23}}=0,3\left(mol\right)\\ 2,m_{HCl}=n.M=0,5.36,5=18,25\left(g\right)\\ 2,n_{N_2}=\dfrac{V_{\left(đkc\right)}}{24,79}=\dfrac{12,395}{24,79}=0,5\left(mol\right)\\ m_{N_2}=n.M=0,5.28=14\left(g\right)\\ d_{\dfrac{O_2}{kk}}=\dfrac{M_{O_2}}{M_{kk}}=\dfrac{32}{29}\)
Câu 5:
\(1,3Fe+2O_2\underrightarrow{t^o}Fe_3O_4\\ 2,Fe+2HCl\rightarrow FeCl_2+H_2\\ 4,2KClO_3\underrightarrow{t^o}2KCl+3O_2\)























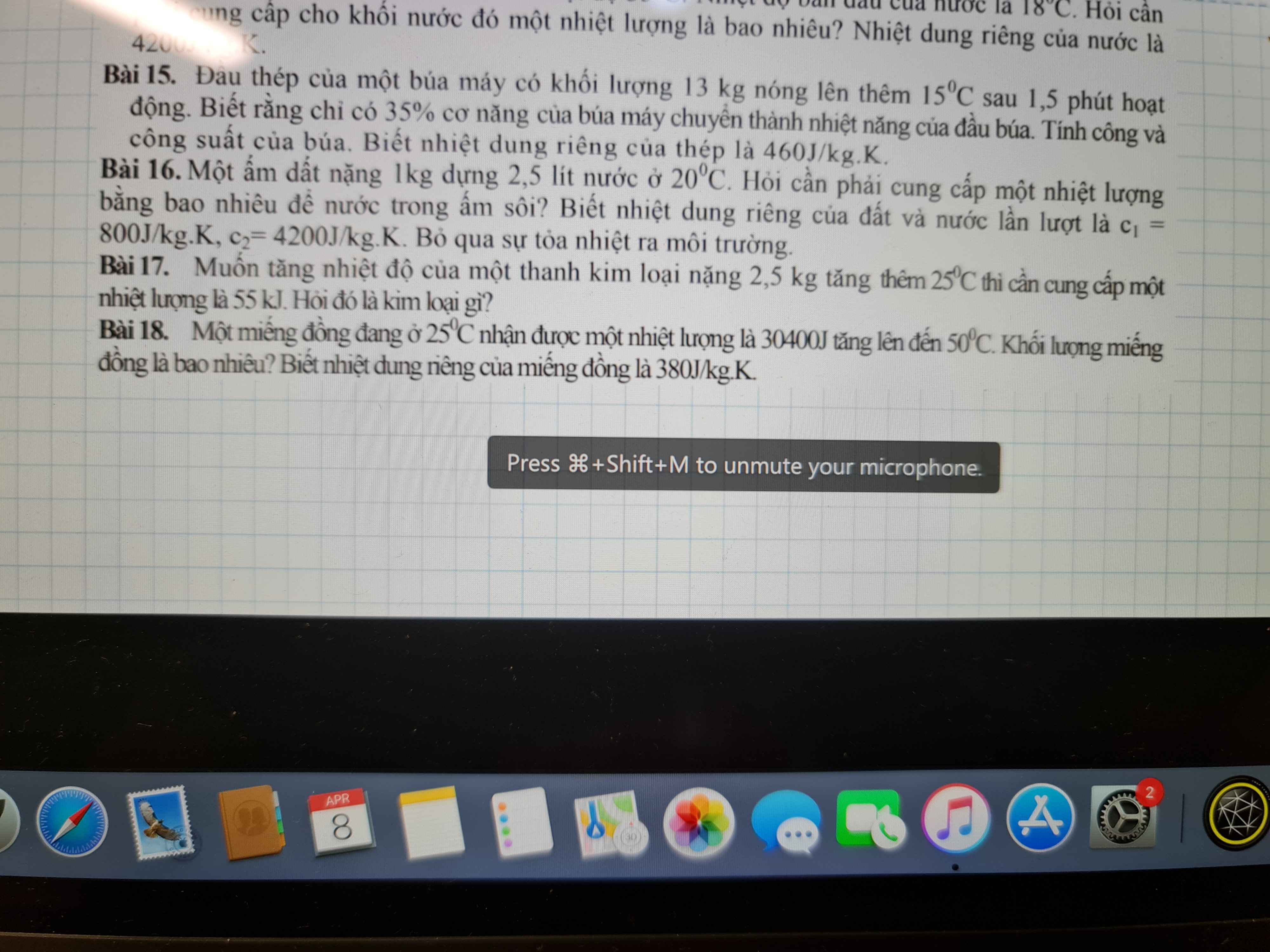

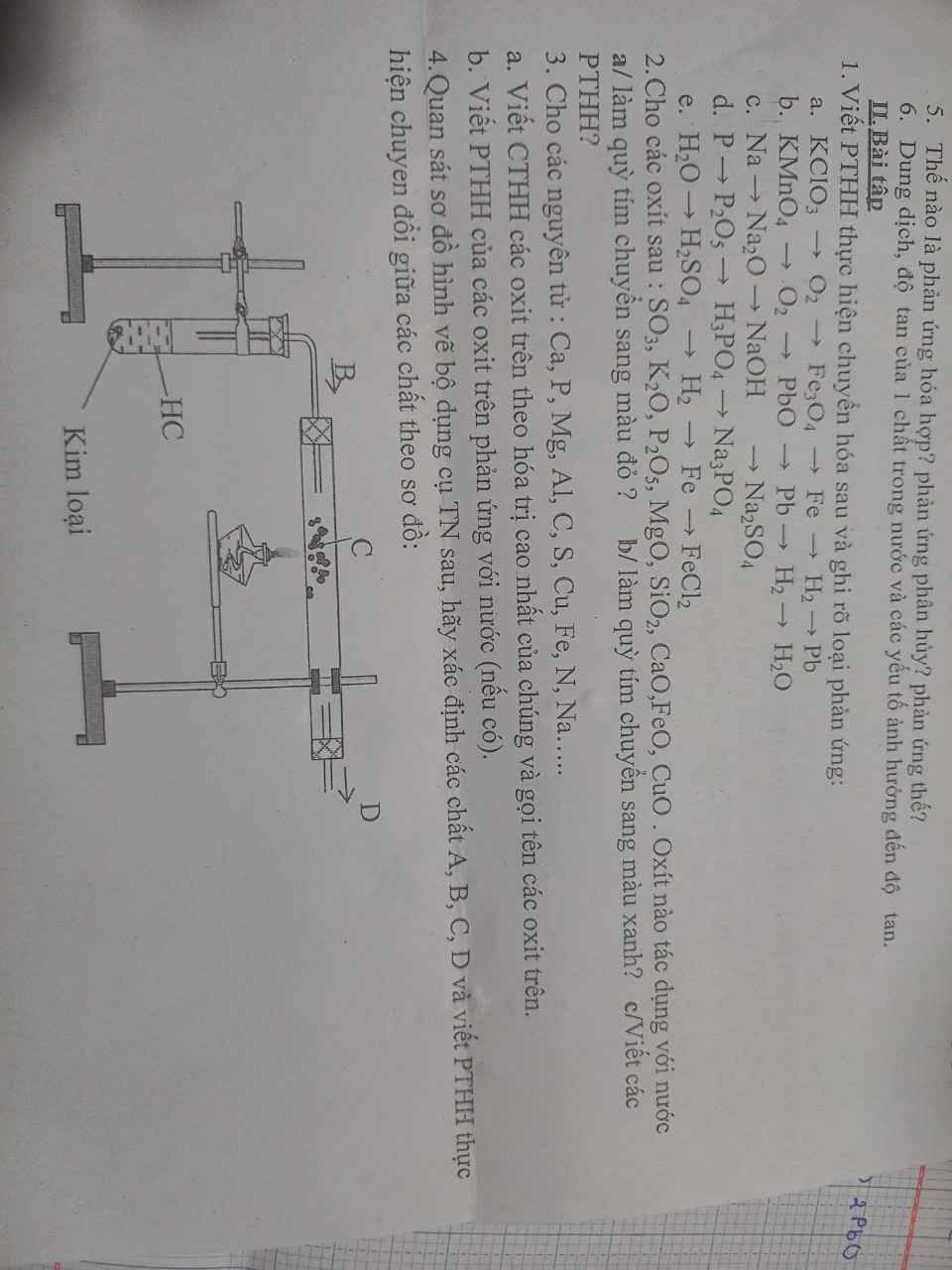 mn giải giúp em với ạ em đag cần gấp , em cảm ơn
mn giải giúp em với ạ em đag cần gấp , em cảm ơn