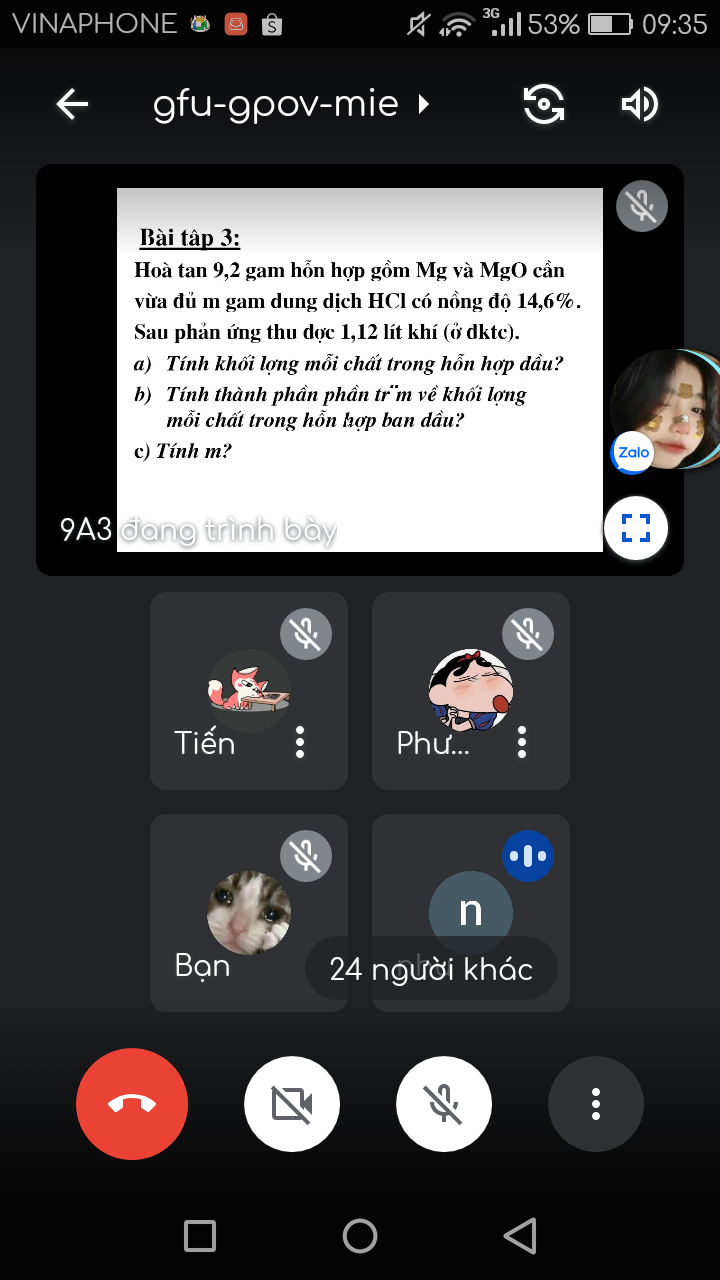
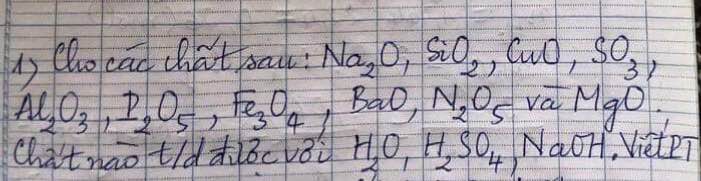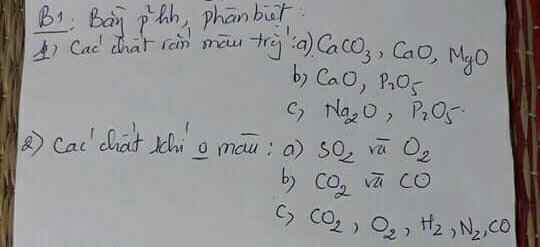Ta có: \(n_{H_2}=\dfrac{1,12}{22,4}=0,05\left(mol\right)\)
PTHH:
Mg + 2HCl ---> MgCl2 + H2 (1)
MgO + 2HCl ---> MgCl2 + H2O (2)
a. Theo PT(1): \(n_{Mg}=n_{H_2}=0,05\left(mol\right)\)
\(\Rightarrow m_{Mg}=0,05.24=1,2\left(g\right)\)
\(\Rightarrow m_{MgO}=9,2-1,2=8\left(g\right)\)
b. Từ câu a, suy ra:
\(\%_{m_{Mg}}=\dfrac{1,2}{9,2}.100\%=13,04\%\)
\(\%_{m_{MgO}}=100\%-13,04\%=86,96\%\)
c. Ta có: \(n_{MgO}=\dfrac{8}{40}=0,2\left(mol\right)\)
\(\Rightarrow n_{hh}=0,2+0,05=0,25\left(mol\right)\)
Theo PT(1,2): \(n_{HCl}=2.n_{hh}=2.0,25=0,5\left(mol\right)\)
\(\Rightarrow m_{HCl}=0,5.36,5=18,25\left(g\right)\)
Ta có: \(C_{\%_{HCl}}=\dfrac{18,25}{m_{dd_{HCl}}}.100\%=14,6\%\)
\(\Rightarrow m=m_{dd_{HCl}}=125\left(g\right)\)